SAN DIEGO, CA--(Marketwire - Jun 8, 2011) - Cytori Therapeutics (
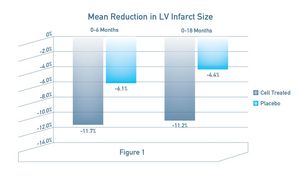
Mean reduction in infarct size at 6 months was preserved at 18 months for the cell treated group (standard-of-care plus cells), at 11.7% and 11.2%, respectively. In contrast, patients receiving control (standard-of-care plus placebo), exhibited diminished treatment effect from 6 months (6.1%) to 18 months (4.4%) (see Fig. 1);
The mean reduction in left ventricular infarct size (reduction in the portion of the heart damaged by the heart attack) was statistically significant (p < 0.05) from baseline to 18 months in the cell-treated group. In patients receiving control, there was less improvement which was not statistically significant;
Statistically significant (p < 0.05) improvement in perfusion of the left ventricle (blood flow through the tissue of the heart) from baseline to 18 months was observed in the cell-treated group. In patients receiving control treatment, there was less improvement which was not statistically significant; and
There were substantially fewer premature ventricular beats (PVBs) per holter recording in patients who had received cells compared to those who had received control (mean of 24 and 146 PVBs per recording, respectively).
"There is a strong correlation between infarct size and clinical outcomes," said Henricus Duckers, M.D., Ph.D., Interventional Cardiologist, Head of Molecular Cardiology, Thoraxcenter, Erasmus University Hospital. "The APOLLO data demonstrate there was a persistent beneficial effect from the cells that we believe if repeated in ADVANCE should translate into superior outcomes for patients. This is very encouraging for the field of cardiac cell therapy given that this is one of the first randomized and double-blind trials to demonstrate sustained improvement in outcomes in heart attack patients as far out as 18 months."
"Heart disease has been the leading killer for more than 100 years and is one of the greatest financial burdens on the healthcare system," said Christopher J. Calhoun, chief executive officer for Cytori. "Reductions of this magnitude in infarct size, if shown to be reproducible in a larger trial would beneficially impact long-term outcomes for patients, including MACCE, the development of chronic ischemia and death. While APOLLO was a safety and feasibility trial, Cytori's larger European trial ADVANCE is designed to demonstrate efficacy, economic impact and the long-term benefit in patient outcomes in up to 370 patients."
As part of this novel procedure, a small amount of fat tissue was removed from each patient's abdomen via liposuction shortly after his or her heart attack. Using a proprietary medical device, the Celution® System developed by Cytori, stem and regenerative cells were rapidly separated from each patient's fat tissue and concentrated at the point-of-care, in the catheter laboratory. The cells were then immediately prepared and infused into the patient's coronary artery. All patients were treated within one day after the successful revascularization of the infarcted artery that caused the heart attack.
As previously reported, the procedure was found to be safe. There were no new major adverse cardiac or coronary events (MACCE) reported between the six and 18 month follow-up. Two MACCE events were reported between baseline and the six month follow up, neither of which was attributed to the procedure. Additionally, at the time of injection, no effect on TIMI flow or coronary flow reserve was noted during and after infusion of cells or placebo, suggesting little or no micro-vascular obstruction by the infused cells.
These new long-term data will be presented on Friday, June 10 at the 8th International Symposium on Stem Cell Therapy & Cardiovascular Innovation in Madrid, Spain by Dr. Duckers, Co-Principal Investigator for the trial. All MRI data were analyzed by an independent blinded core laboratory. The core laboratory remained blinded to patients' treatment assignments through 18 months.
The APOLLO trial is led by co-Principal Investigators Patrick W. Serruys, M.D., Ph.D., Professor of Interventional Cardiology at the Thoraxcenter, Erasmus University Hospital and Dr. Duckers. Francisco F. Avilés, M.D., Ph.D., Professor of Medicine and Chief of Department of Cardiology at Hospital General Universitario Gregorio Marañón in Madrid also participated in this trial.
Cytori is currently enrolling patients in the pivotal ADVANCE trial in Europe, designed to treat the same ST-elevation acute heart attack patients as in the APOLLO trial. ST-elevation heart attacks are a severe form of heart attack in which blood supply is cut off from the heart for a prolonged period of time, impacting a large portion of the heart muscle. Approximately 1.3 million patients in the United States and 1.9 million in Europe suffer from acute heart attacks each year.
About Cytori
Cytori is a leader in providing patients and physicians around the world with medical technologies that harness the potential of adult regenerative cells from adipose tissue. The Celution® System family of medical devices and instruments is being sold into the European and Asian cosmetic and reconstructive surgery markets but is not yet available in the United States. Our StemSource® product line is sold globally for cell banking and research applications. Our PureGraft™ products are available in North America and Europe for fat grafting procedures. www.cytori.com
Cautionary Statement Regarding Forward-Looking Statements
This press release includes forward-looking statements regarding events, trends, business prospects and particularly the APOLLO clinical study results, which may affect our future operating results and financial position. Such statements, including, but not limited to, those regarding improvements in patient outcomes, the significance of the physiological and functional effects from the pilot APOLLO study, and the effectiveness of the design of the ADVANCE study, are all subject to risks and uncertainties that could cause the results of the more comprehensive ADVANCE study to differ materially from those presented above. Some of these risks and uncertainties include, but are not limited to, risks related to the statistical power of the APOLLO study, the inherent risk and uncertainty in the costs and potential variability of outcomes of a pivotal heart attack study, uncertainties regarding the collection and results of clinical data, and dependence on third party performance, as well as other risks and uncertainties described under the "Risk Factors" in Cytori's Securities and Exchange Commission Filings on Form 10-K and Form 10-Q. We assume no responsibility to update or revise any forward-looking statements to reflect events, trends or circumstances after the date they are made.
Contact Information:
Contact
Investors:
Tom Baker
tbaker@cytori.com
+1.858.875.5258
US Media:
Megan McCormick
mmccormick@cytori.com
+1.858.875.5279
European Media:
Gemma Howe
College Hill
Gemma.howe@collegehill.com
+44 20 7866 7860