FORT LAUDERDALE, Fla., Dec. 11, 2012 (GLOBE NEWSWIRE) -- Imaging Diagnostic Systems, Inc. (OTC:IMDSD), a pioneer in laser breast imaging, announced today that Clinical Imaging, a leading US based radiologist peer review journal, has reviewed and accepted a paper that evaluates the results of CTLM® and mammography when imaging dense breasts.
The recent paper accepted by Clinical Imaging focuses on imaging women with heterogeneously and extremely dense breast tissue using both mammography and CTLM®. CTLM® is an innovative 3D laser breast imaging system that utilizes Diffuse Optical Tomography or "DOT" technology. The study demonstrated that the addition of a CTLM® scan to a mammogram improved sensitivity rates of detecting breast abnormalities considerably. The sensitivity rates ranged from 34.4% for mammography alone to 81.57% when CTLM® was added. Equating to over double the sensitivity to detect abnormalities when imaging extremely dense breasts (ACR BIRADS 4 breast composition classification). To access the free abstract and the complete clinical paper for purchase, please go to www.clinicalimaging.org and search under the heading for either "CTLM" or "CTLM as an adjunct to mammography in the diagnosis of patients with dense breast". Globally, 40-50% of the female population have mammographically dense breasts and these recent results present the potential of CTLM® to assist the imaging needs of women with any breast density.
Clinical Imaging provides widespread coverage of innovative technology, new applications, and important issues concerning all diagnostic imaging techniques. The journal investigates the relative merits of established and developing diagnostic imaging technology, with regard to cost effectiveness, safety, and propriety where specific disorders and physiological systems are concerned. Clinical Imaging is a radiologist peer reviewed publication. From ultrasound to MRI, Clinical Imaging provides crucial information for radiologists, radiology residents, and radiologic technologists.
The paper's author, Dr. Jin Qi remarks, "Our data indicated that the imaging of CTLM® was least affected by tissue density in breasts and provides information about angiogenesis in breast lesions, especially in malignant lesions, when used as an adjunct to mammography in heterogeneously dense breasts and extremely dense breasts, sensitivity increased significantly." Dr. Qi concluded, "this is a feature that could have a positive impact on women's imaging across the globe." Dr. Qi is deeply involved with breast cancer in China and works in the following departments; Radiology Department at Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Breast Cancer Prevention and Therapy of the Ministry of Education, and Key Laboratory of Cancer Prevention and Therapy.
CEO of Imaging Diagnostic Systems, Inc., Linda Grable states, "IDSI is very excited and honored to have a CTLM® paper accepted by a leading, well respected American diagnostic imaging publication. The results display the capability of CTLM® to be less impeded by dense breast tissue than mammography and will eventually provide radiologists with a complimentary non radiation based imaging tool; especially when dealing with dense breast tissue. And the additional patient benefits consisting of no radiation, no breast compression, and no injected contrast agents will hopefully aide with women's imaging needs globally".
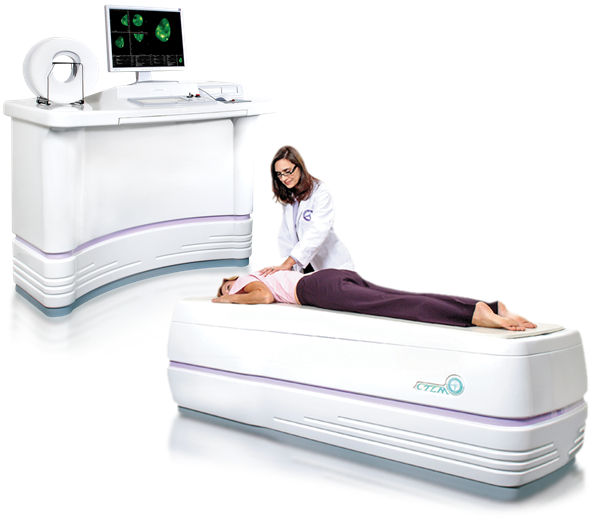
Imaging Diagnostic Systems, Inc. is the developer of the CTLM® system; a revolutionary optical breast imaging device. Optical mammography is a diffuse optical tomography (DOT) technique that aims at detecting breast cancer. The CTLM® system utilizes patented continuous wave laser technology and computed algorithms to create 3D images of the breast. The procedure is non-invasive, painless, and does not expose the patient to ionizing radiation or painful breast compression. The CTLM® system has received certification and licenses to sell internationally through the following approvals; European CE marking, Health Canada, China SFDA, UL, ISO 13485:2003, FDA export certification. Marketing clearances pending are Mexico COFEPRIS and Russian GOST-R.
For more information, visit our website: www.imds.com
The Imaging Diagnostic Systems, Inc. logo is available at http://www.globenewswire.com/newsroom/prs/?pkgid=16214
As contemplated by the provisions of the Safe Harbor section of the Private Securities Litigation Reform Act of 1995, this news release may contain forward-looking statements pertaining to future, anticipated, or projected plans, performances and developments, as well as other statements relating to future operations. All such forward-looking statements are necessarily only estimates or predictions of future results or events and there can be no assurance that actual results or events will not materially differ from expectations. Further information on potential factors that could affect Imaging Diagnostic Systems, Inc. is included in the Company's filings with the Securities and Exchange Commission. We expressly disclaim any intent or obligation to update any forward-looking statements.
Contact: Investor Relations, 954-581-9800
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=16212