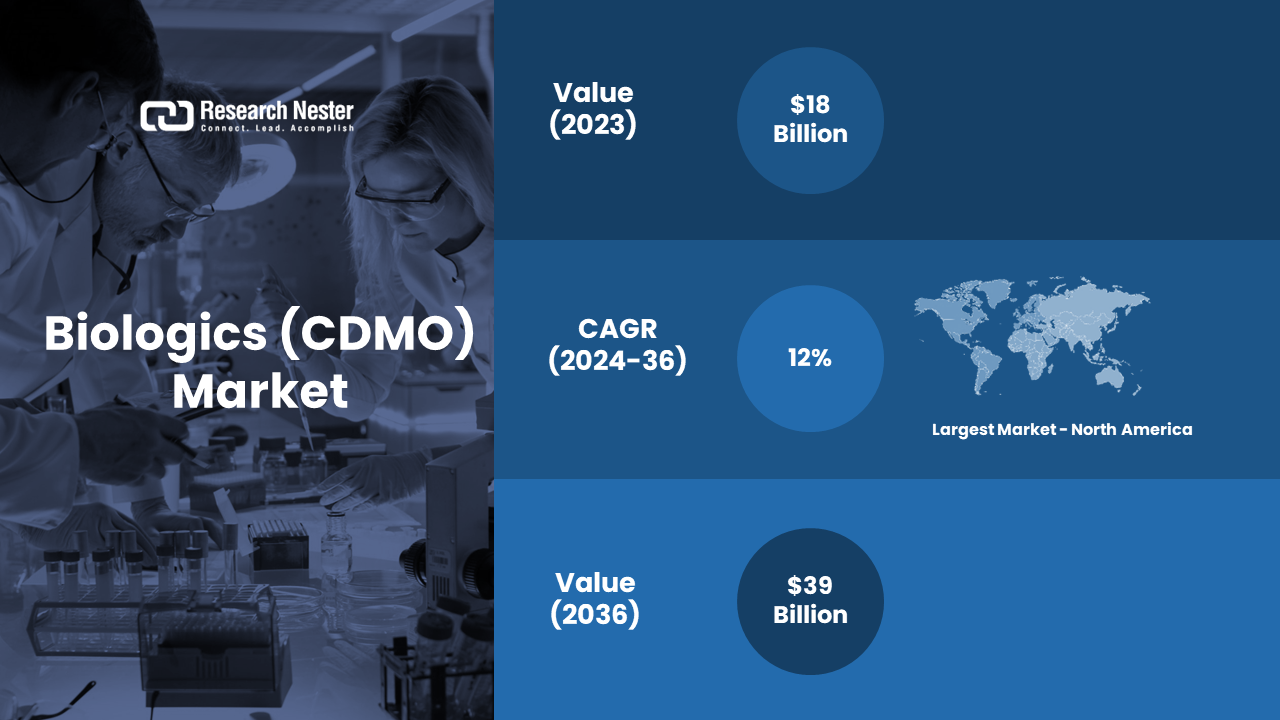
New York, Feb. 08, 2024 (GLOBE NEWSWIRE) -- The global biologics contract development & manufacturing organization (CDMO) market size is projected to grow at a CAGR of over ~12% from 2024 to 2036. The market is expected to garner a revenue of USD 39 billion by the end of 2036, up from a revenue of ~USD 18 billion in the year 2023.The number of diabetes patients is set to rise to about 642 million by 2030 up from a number of over 536 million in the year 2021. Further, by 2045 the number of patients is estimated to reach approximately 782 million. Hence, the demand for biologics CDMO is set to grow.
Request Free Sample Copy of this Report @ https://www.researchnester.com/sample-request-5516
Additionally, there has been a surge in outsourcing of clinical services. While traditional underlying drivers such as the availability of specialized knowledge and technology (exacerbated by treatments being more complex), cost advantages, and a greater focus on marketing and innovation among big. Pharma remains strong, but significant recent developments have occurred that strengthen the need for outsourcing investment. Some of these recent developments include automation, the Internet of Things (IoT), software development, and other contemporary technologies. However, this technology needs to be updated recurrently which is why the demand for outsourcing is growing. Hence, the biologics contract development & manufacturing organization (CDMO) market revenue is poised to rise. Cell and gene therapies (CGTs) are going to keep multiplying as long as CDMOs continue to expand the sector and leave their mark on human history.
Increasing COVID-19 Cases across the Globe to Boost Market Growth
Worldwide coronavirus (COVID-19) cases were close to 767 million as of 2023. Hence, the demand for CDMO has been surging since the COVID-19 pandemic has brought attention to how essential it is to have a robust and reliable supply chain for the development and production of biologics. In addition, the increasing geopolitical tensions with China and other regions of the world have merely made it more important to have an efficient supply chain management system. This system is established to reduce any supply risks that could negatively impact the personnel, equipment, and raw material quantity and accessibility required for the manufacture of biologics. Ageing is a challenging process that involves a multisystem functional decline, flaws in many homeostatic processes, and a dramatically diminished ability to adapt to stress. Furthermore, the pharmacokinetics of pharmaceuticals are modified in the elderly population, leading to elevated hazards associated with their usage. These modifications are associated with an increase in total body fat and reductions in lean body mass, total body water, serum albumin level, and renal and hepatic function. Consequently, there is a higher risk of myelosuppression, cardiotoxicity, renal insufficiency, and neurotoxicity in older individuals using drugs.
Biologics Contract Development & Manufacturing Organization (CDMO) Market: Regional Overview
The market is segmented into five major regions including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa region.
Increasing Demand for Biopharmaceuticals to Drive the Market Growth in North America Region
The biologics contract development & manufacturing organization (CDMO) market in North America region is estimated to garner the largest revenue by the end of 2036. The major factor to dominate the market share in this region is rising pharmaceutical companies. The USA holds a special distinction of being the world's largest manufacturer of pharmaceuticals. Due to the rapid technological breakthroughs occurring in the nation, some experts even rank it as the best country for the pharmaceutical business. Particularly, the United States of America tops the list of nations that import the most pharmaceuticals, underscoring its important position in the world pharmaceutical industry. Hence, the market for biologic CDMO is also surging in this region. Furthermore, this region is observing a huge rise in clinical trials which has further led to a shift from academic medical centers to community-based practices which is further predicted to dominate the biologics contract development & manufacturing organization (CDMO) market growth in this region. The rise in chronic diseases, coupled with advancements in biotechnology, has led to an expanded pipeline of biologics in development. Consequently, pharmaceutical companies are increasingly outsourcing their manufacturing processes to specialized CDMOs, driving the market's growth. According to a report, the United States alone accounted for approximately 45% of the global biopharmaceutical research and development spending in 2020.
Make an Inquiry Before Buying this Report @ https://www.researchnester.com/inquiries-before-buying-5516
Growing Population to Propel the Growth in the Asia Pacific Region
The Asia Pacific biologics contract development & manufacturing organization (CDMO) market is estimated to garner the highest CAGR by the end of 2036. One of the primary drivers for the growth of the market in the Asia Pacific region is the cost advantage offered by manufacturers in this geography. The cost of labor, facilities, and raw materials is comparatively lower than in Western countries. This cost-effectiveness has resulted in a substantial shift of biopharmaceutical manufacturing activities to the Asia Pacific. This growth is further set to be influenced by the rising population. 60 percent of the global population, which is approximately 4.3 billion people, live in the Asia and Pacific region, which happens to be home to China and India, the two most populous nations on earth. Furthermore, there has been a rise in government programs to spread awareness regarding the availability of biologics and influence the imports of various biologics. This is why the market for biologics CDMO is rising in this region.
Biologics Contract Development & Manufacturing Organization (CDMO), Segmentation by Cell Line Type
- Microbial
- Mammalian
- Viral Vector & Other Modalities
Amongst these segments, the mammalian segment in biologics (CDMO) market is anticipated to hold the largest share over the forecast period. When producing biopharmaceuticals, mammalian cell lines are frequently employed due to their precision in replicating complicated proteins, which ensures high bioactivity and low post-translational changes. This sort of cell is particularly useful for generating monoclonal antibodies, which make up a significant number of biological medications. Scalability, dependability, and comparability with the complex needs of therapeutic protein production are offered by mammalian cell cultures. The dominance of mammalian cell culture technology highlights its critical role in satisfying the expanding demand for sophisticated biopharmaceuticals on a global basis as the biologics CDMO industry continues to rise. The mammalian segment of the biologics contract development and manufacturing organization (CDMO) market has experienced substantial growth, driven primarily by the heightened demand for monoclonal antibodies (mAbs). According to a report from the Biotechnology Innovation Organization (BIO), the global sales of monoclonal antibodies reached over USD 150 billion in 2020.
Biologics Contract Development & Manufacturing Organization (CDMO), Segmentation by Product Type
- Biologics
- Biosimilars
Amongst these segments, the biologics segment in biologics (CDMO) market is anticipated to hold a significant share over the forecast period. The expanding pipeline of biopharmaceuticals, including monoclonal antibodies, vaccines, and therapeutic proteins, has been a major driver for the growth of the biologics segment. Pharmaceutical companies continue to invest heavily in research and development (R&D) to bring novel biologics to market. According to a report by the Pharmaceutical Research and Manufacturers of America (PhRMA), there were over 1,100 biologics in development globally in 2020, covering a diverse range of therapeutic areas. As the biopharmaceutical pipeline diversifies and matures, CDMOs specializing in biologics play a crucial role in supporting the development, scale-up, and manufacturing of these innovative therapies. The continuous advancements in biotechnology and genomics have been pivotal in driving growth within the biologics segment. Techniques such as CRISPR-Cas9 gene editing and high-throughput screening have revolutionized the discovery and development of biologics by enabling the creation of highly specific and targeted therapeutic agents. The shift towards personalized medicine, tailoring treatments based on individual patient characteristics, has significantly influenced the growth of the biologics segment. Biologics, with their ability to target specific molecular pathways and biomarkers, align well with the principles of personalized medicine.
Few of the well-known industry leaders in biologics contract development & manufacturing organization (CDMO) market that are profiled by Research Nester are AGC Biologics, Biocon Biologics Ltd., Catalent, Inc., Emergent BioSolutions, Thermo Fisher Scientific Inc., and other key market players.
Recent Development in the Biologics (CDMO) Market
- At its protein biologics manufacturing facility in Seattle, AGC Biologics, a preeminent worldwide Biopharmaceutical Contract Development and Manufacturing Organisation (CDMO), has announced the launch of a new commercial manufacturing project. Provention Bio, Inc., a biopharmaceutical firm dedicated to furthering the development of investigational medicines that may intercept and prevent debilitating and life-threatening immune-mediated disorders, is the source of TZIELD (teplizumab-mzwv), a novel medication for Type 1 Diabetes that the CDMO is creating.
- Being the first provider of biosimilar insulins to endorse and support the International Diabetes Federation (IDF) and its Core Mission initiative, Biocon Biologics Ltd. is a fully integrated, "pure play" biosimilars firm and a subsidiary of Biocon Ltd.
Read our insightful Blogs and Data-driven Case Studies:
- Precision Medicine - A Tailor-Made Targeted Therapy of the Future, Revolutionizing the Healthcare Industry
Explore the mechanisms, methodologies and benefits of precision medicine, a healthcare approach that considers an individuals distinct genetic composition, surroundings and lifestyle to create customized treatments.
- Pharma Company proactively introduced NASH Therapeutics Products for revenue increment
Know how a pharmaceutical company increased its market share using our product analysis solution. We offered research on NASH treatments which helped them learn market players’ products and other insights.
About Research Nester
Research Nester is a one-stop service provider with a client base in more than 50 countries, leading in strategic market research and consulting with an unbiased and unparalleled approach towards helping global industrial players, conglomerates and executives for their future investment while avoiding forthcoming uncertainties. With an out-of-the-box mindset to produce statistical and analytical market research reports, we provide strategic consulting so that our clients can make wise business decisions with clarity while strategizing and planning for their forthcoming needs and succeed in achieving their future endeavors. We believe every business can expand to its new horizon, provided a right guidance at a right time is available through strategic minds.
