Dublin, Feb. 09, 2024 (GLOBE NEWSWIRE) -- The "Global Clinical Trials Market by Phase (Phase I, II, III), Service Type (Laboratory, Analytical Testing, Patient Recruitment, Protocol Designing), Therapeutic Area (Oncology, Cardiology, Neurology), and Application (Vaccine, mAbs, CGT) - Forecast to 2028" report has been added to ResearchAndMarkets.com's offering.
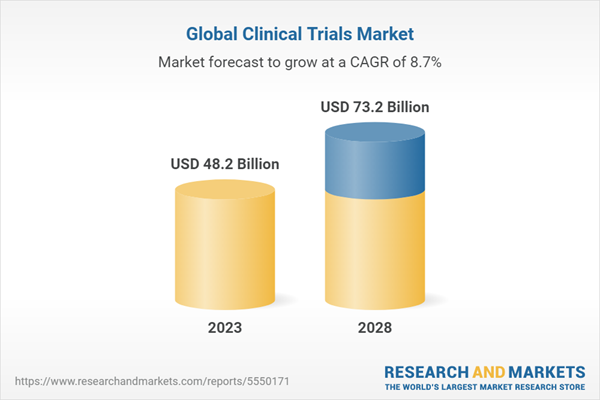
The global clinical trials market is projected to reach USD 73.2 billion by 2028 from USD 48.2 billion in 2023, at a CAGR of 8.7% during the forecast period of 2023 to 2028. The growth of this market can be attributed to the increasing demand for specialized testing services. Companies are increasingly outsourcing specialized testing services, such as liquid chromatography-mass spectrometry (LC/MS), RNA sequencing, gene expression analysis, wet chemistry analysis of compendia raw materials, and trace metal analysis with inductively coupled mass spectrometry (ICP-MS). These tests require advanced equipment and skilled professionals. In an effort to reduce equipment and labor expenses, pharmaceutical and biopharmaceutical enterprises prefer the outsourcing of these specialized testing services to Contract Research Organizations (CROs).
This research report categorizes the clinical trials market by phase, service type, therapeutic area, application and region. The scope of the report covers detailed information regarding the major factors, such as drivers, trends, challenges, and opportunities, influencing the growth of the clinical trials market. A detailed analysis of the key industry players has been done to provide insights into their business overview, solutions, services, key strategies, collaborations, partnerships, and agreements. New launches, mergers and acquisitions, and recent developments associated with the clinical trials market.
The small molecules segment accounted for the largest share by application in 2022
In 2022, the small molecules segment accounted for the largest share by application in the global clinical trials market. Small molecules cover a wide spectrum of therapeutic areas, from cardiovascular diseases to infectious diseases to cancer. This versatility allows for a diverse range of clinical trials across different medical conditions. The development process for small molecules is well-established and understood by regulatory agencies like the US FDA. This familiarity expedites the approval process and encourages pharmaceutical companies to invest in clinical trials for these compounds. These factors contributed to the dominance of the segment in 2022.
The US has continued to dominate the clinical trials market during the forecast period of 2023-2028
The US dominated the clinical trials market in North America in 2022. The major share of the US clinical trials market can be attributed to the significant number of clinical trials and drug discovery activities conducted in the country, coupled with the presence of state-of-the-art research and development infrastructure within the pharmaceutical and biopharmaceutical sectors. The US serves as a global hub for clinical trials, with a significant portion of drug development research being carried out in the US. This trend is attributed to its robust regulatory framework and highly advanced hospital infrastructure. According to ClinicalTrials.gov, as of August 2023, the global count of registered clinical studies reached 463,610. Notably, one-third of this total, accounting for 165,148 registered clinical studies, were concluded within the US.
List of Companies Profiled in the Report
- IQVIA Inc. (US)
- Laboratory Corporation of America Holdings (US)
- Syneos Health (US)
- WuXi AppTec (China)
- Charles River Laboratories (US)
- Parexel International Corporation (US)
- Thermo Fisher Scientific Inc. (US)
- ICON plc (Ireland)
- Medpace (US)
- ACM Global Laboratories (US)
- Advanced Clinical (US)
- SGS (Switzerland)
- Frontage Labs (US)
- PSI (Switzerland)
- Bioagile (India)
- Fortrea Inc. (US)
- Clinical Trial Service (Netherlands)
- Worldwide Clinical Trials (US)
- Pepgra (UK)
- CTI Clinical Trial & Consulting (US)
- Dove Quality Solutions (UK)
- Firma Clinical Research (US)
- Celerion (US)
- Novotech (Australia)
- Clinical Americas (Japan)
Key Attributes
| Report Attribute | Details |
| No. of Pages | 342 |
| Forecast Period | 2023 - 2028 |
| Estimated Market Value (USD) in 2023 | $48.2 Billion |
| Forecasted Market Value (USD) by 2028 | $73.2 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
Market Dynamics
- Drivers
- Increasing Drugs in Pipeline and Rising Investments in Pharmaceutical R&D
- Increasing Number of Clinical Trials
- High Cost of In-House Drug Development
- Rising Prevalence of Orphan and Rare Diseases
- Opportunities
- Favorable Outlook for Biologics and Biosimilars
- Rising Demand for Specialized Testing Services
- Need for Novel Clinical Trial Designs for Complex Cell and Gene Therapies
- Challenges
- Shortage of Skilled Professionals for Clinical Trials
- Need for Unique Testing Approaches for Innovative Molecules
- Trends
- Adoption of AI-based Tools for Drug Discovery
- Increasing Outsourcing Activities in Emerging Asian Economies
- Integrated End-to-End R&D Service Solutions
For more information about this report visit https://www.researchandmarkets.com/r/nm1o43
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
