New York, USA, May 14, 2024 (GLOBE NEWSWIRE) -- Bladder Cancer Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 70+ Companies Working in the Domain
Bladder cancer, marked by abnormal cell growth in the bladder lining, presents a global health concern. The incidence of bladder cancer is on the rise due to aging populations, necessitating increased demand for diagnostics and therapies. Technological advancements and awareness campaigns are playing important roles in early detection, thereby augmenting the market. With growing healthcare expenditure, there's a significant push towards research and personalized treatment options, promising better outcomes for patients. Additionally, expansion into emerging markets is fostering demand for advanced solutions, highlighting a global need for improved bladder cancer management strategies.
DelveInsight’s 'Bladder Cancer Pipeline Insight 2024' report provides comprehensive global coverage of pipeline bladder cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the bladder cancer pipeline domain.
Key Takeaways from the Bladder Cancer Pipeline Report
- DelveInsight’s bladder cancer pipeline report depicts a robust space with 70+ active players working to develop 80+ pipeline therapies for bladder cancer treatment.
- Key bladder cancer companies such as UroGen Pharma, HiberCell, BiVictriX Therapeutics, Deka Biosciences, BeyondSpring Pharmaceuticals, Lipella Pharmaceuticals, Asieris Pharmaceuticals, G1 Therapeutics Inc., LintonPharm Co.Ltd., Vaxiion Therapeutics, Merck Sharp & Dohme LLC, CG Oncology, Inc., SURGE Therapeutics, ImmunityBio, Inc., enGene, Inc., CicloMed LLC, Protara Therapeutics, Hoffmann-La Roche, CG Oncology, Inc., Astellas Pharma Global Development, Inc, Akamis Bio, Prokarium, and others are evaluating new bladder cancer drugs to improve the treatment landscape.
- Promising bladder cancer pipeline therapies such as UGN-301, HC 7366, BVX-003, DK 15-10, Plinabulin, LP-50, APL 1202, Trilaciclib, Catumaxomab, VAX 014, Pembrolizumab, CG0070, STM-416, BCG+N-803, EG-70, CPX-POM, TARA-002, Atezolizumab, CG0070, Enfortumab vedotin, Enadenotucirev, ZH 9, and others are under different phases of bladder cancer clinical trials.
- In February 2024, CG Oncology commenced the Phase III PIVOT-006 clinical trial of cretostimogene for the treatment of intermediate-risk non-muscle invasive bladder cancer (NMIBC) with the dosing of the first subject.
- In February 2024, ImmunityBio announced that findings from Patient-Reported Outcomes (PROs) of participants in the phase II/III QUILT 3.032 study of N-803 plus BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) were published by the peer-reviewed journal Urology Practice. These PROs support the positive interim results from the study published in NEJM Evidence, wherein 71% of patients in cohort A with CIS with or without Ta/T1 disease achieved a complete response.
- In February 2024, UK-based biopharmaceutical company Prokarium dosed the first subject in the PARADIGM-1 Phase I/Ib study of ZH9, an investigational bacterial immunotherapy, for patients with non-muscle invasive bladder cancer (NMIBC) in the US.
- In January 2024, the FDA granted fast track and breakthrough therapy designation to cretostimogene grenadenorepvec (CG0070) for use as a potential therapeutic option in patients with high-risk Bacillus Calmette-Guérin (BCG)–unresponsive non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ with or without Ta or T1 tumors.
- In January 2024, Canadian pharmaceutical company Theralase Technologies released interim clinical data from its Phase II study of its intravesical photodynamic therapy Ruvidar (TLD-1433) to treat patients with non-muscle invasive bladder cancer.
- In December 2023, Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) has granted TAR-200 Breakthrough Therapy Designation (BTD) for the potential future treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (HR-NMIBC), who are ineligible for or elected not to undergo radical cystectomy (surgical removal of the bladder).
- In October 2023, CatalYm had dosed the first subject in the Phase II GDFather-NEO clinical trial of visugromab (CTL-002) along with nivolumab to treat patients with muscle-invasive bladder cancer (MIBC).
Request a sample and discover the recent advances in bladder cancer treatment drugs @ Bladder Cancer Pipeline Report
The bladder cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage bladder cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the bladder cancer clinical trial landscape.
Bladder Cancer Overview
Bladder cancer is a condition that typically initiates in the cells lining the inner walls of the bladder. It commonly impacts individuals over 70 years old and is more prevalent in men. It ranks as the fifth most prevalent cancer type overall and the fourth most frequent in men. Timely diagnosis often yields positive responses to treatment. However, individuals successfully treated for bladder cancer should undergo regular monitoring thereafter.
In most instances, the initial indication of bladder cancer is the presence of blood in the urine. Additional symptoms may involve experiencing pain or a burning sensation during urination, or alterations in urination patterns, such as frequent urination or the urge to urinate with difficulty passing urine. Advanced stages of bladder cancer might entail symptoms such as one-sided lower back pain, fatigue, weakness, loss of appetite, and weight loss. The primary risk factor for developing bladder cancer is tobacco use. Smokers are up to four times more likely than nonsmokers to develop this condition, with smoking accounting for approximately half of all cases.
Diagnostic methods for bladder cancer include procedures such as cystoscopy to examine the bladder's interior, biopsy, urine cytology, and imaging tests for confirmation and locating cancerous growths elsewhere in the body. These imaging tests may include CT scans, MRIs, PET scans, bone scans, and chest X-rays. Treatment approaches for bladder cancer are contingent on various factors, including the cancer type, grade, and stage, in addition to the patient's overall health. Options for bladder cancer treatment may encompass surgery for the removal of cancerous cells, intravesical chemotherapy, systemic chemotherapy, radiation therapy as a primary treatment method, immunotherapy to stimulate the body's immune system against cancer cells, and targeted therapy for advanced cases.
Find out more about bladder cancer treatment drugs @ Drugs for Bladder Cancer Treatment
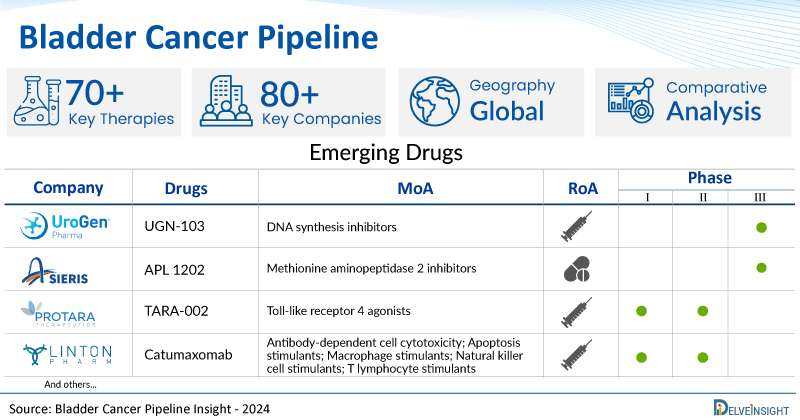
A snapshot of the Bladder Cancer Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| UGN-103 | UroGen Pharma Ltd. | Phase III | DNA synthesis inhibitors | Intravesical |
| APL 1202 | Asieris Pharmaceuticals | Phase III | Methionine aminopeptidase 2 inhibitors | Oral |
| TARA-002 | Protara Therapeutics | Phase I/II | Toll-like receptor 4 agonists | Intravesical |
| Catumaxomab | LintonPharm Co., Ltd. | Phase I/II | Antibody-dependent cell cytotoxicity; Apoptosis stimulants; Macrophage stimulants; Natural killer cell stimulants; T lymphocyte stimulants | Intravesical |
| VAX 014 | Vaxiion Therapeutics | Phase I | Apoptosis stimulants; Cell death stimulants; Immunologic cytotoxicity | Intravesical |
Learn more about the emerging bladder cancer pipeline therapies @ Bladder Cancer Clinical Trials
Bladder Cancer Therapeutics Assessment
The bladder cancer pipeline report proffers an integral view of the bladder cancer emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Bladder Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: DNA synthesis inhibitors, Methionine aminopeptidase 2 inhibitors, Cyclin-dependent kinase 4 inhibitors, Cyclin-dependent kinase 6 inhibitors, Antibody-dependent cell cytotoxicity, Apoptosis stimulants, Macrophage stimulants, Natural killer cell stimulants, T lymphocyte stimulants, Cell death stimulants, Immunologic cytotoxicity, Toll-like receptor 4 agonists
- Key Bladder Cancer Companies: UroGen Pharma, HiberCell, BiVictriX Therapeutics, Deka Biosciences, BeyondSpring Pharmaceuticals, Lipella Pharmaceuticals, Asieris Pharmaceuticals, G1 Therapeutics Inc., LintonPharm Co.Ltd., Vaxiion Therapeutics, Merck Sharp & Dohme LLC, CG Oncology, Inc., SURGE Therapeutics, ImmunityBio, Inc., enGene, Inc., CicloMed LLC, Protara Therapeutics, Hoffmann-La Roche, CG Oncology, Inc., Astellas Pharma Global Development, Inc, Akamis Bio, Prokarium, and others
- Key Bladder Cancer Pipeline Therapies: UGN-301, HC 7366, BVX-003, DK 15-10, Plinabulin, LP-50, APL 1202, Trilaciclib, Catumaxomab, VAX 014, Pembrolizumab, CG0070, STM-416, BCG+N-803, EG-70, CPX-POM, TARA-002, Atezolizumab, CG0070, Enfortumab vedotin, Enadenotucirev, ZH 9, and others
Dive deep into rich insights for new drugs for bladder cancer treatment, visit @ Bladder Cancer Drugs
Table of Contents
| 1. | Bladder Cancer Pipeline Report Introduction |
| 2. | Bladder Cancer Pipeline Report Executive Summary |
| 3. | Bladder Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Bladder Cancer Clinical Trial Therapeutics |
| 6. | Bladder Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Bladder Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Bladder Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Bladder Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Bladder Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Bladder Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Bladder Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the bladder cancer pipeline therapeutics, reach out @ Bladder Cancer Treatment Drugs
Related Reports
Bladder Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bladder cancer companies including RemeGen Co., Ltd., Taiho Oncology, Inc., Xennials Therapeutics, Flame Biosciences, among others.
Non-Muscle Invasive Bladder Cancer Pipeline
Non-Muscle Invasive Bladder Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key non-muscle invasive bladder cancer companies, including UroGen Pharma, CG Oncolgy, Sasanlimab, Sesen Bio, Theralase Technologies, Asieris Pharmaceuticals, Hamlet Pharma, AstraZeneca, Viralytics, ImmunityBio, Prokarium, among others.
Non-Muscle Invasive Bladder Cancer Market
Non-Muscle Invasive Bladder Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NMIBC companies, including UroGen Pharma, Pfizer, Roche, CG Oncology, ImmunityBio, Theralase, enGene, SURGE Therapeutics, Aura Biosciences, UroGen Pharma Ltd., FKD Therapies Oy, ImmunityBio, Inc., Protara Therapeutics, Janssen Research & Development, LLC, Tyra Biosciences, Inc, among others.
Muscle-Invasive Bladder Cancer Pipeline
Muscle-Invasive Bladder Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key muscle-invasive bladder cancer companies, including SOTIO a.s., Celon Pharma SA, Shanghai PerHum Therapeutics Co., Ltd., 4D Pharma PLC, among others.
Muscle-Invasive Bladder Cancer Market
Muscle-Invasive Bladder Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key muscle-invasive bladder cancer companies, including SOTIO a.s., Celon Pharma SA, Shanghai PerHum Therapeutics Co., Ltd., 4D Pharma PLC, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
