New York, USA, July 01, 2024 (GLOBE NEWSWIRE) -- Advanced Kidney Cancer Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 50+ Companies Working in the Domain
Advanced kidney cancer refers to a stage of kidney cancer where the disease has spread beyond the kidney to other parts of the body, requiring more complex and aggressive treatment strategies. The Advanced Kidney Cancer market is witnessing robust growth due to the rising prevalence of the disease and significant advancements in treatment options. Innovative therapies, including targeted treatments and immunotherapies, are offering more effective and less toxic alternatives, enhancing patient outcomes. Additionally, improved early detection efforts are driving higher diagnosis rates, further boosting demand for kidney cancer drugs. These factors collectively indicate a promising future for the Advanced Kidney Cancer market.
DelveInsight’s 'Advanced Kidney Cancer Pipeline Insight 2024' report provides comprehensive global coverage of pipeline advanced kidney cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the advanced kidney cancer pipeline domain.
Key Takeaways from the Advanced Kidney Cancer Pipeline Report
- DelveInsight’s advanced kidney cancer pipeline report depicts a robust space with 50+ active players working to develop 55+ pipeline therapies for advanced kidney cancer treatment.
- Key advanced kidney cancer companies such as Janux Therapeutics, Infinity Pharmaceuticals, Merck Sharp & Dohme, Xencor, Exelixis, Nykode Therapeutics, AstraZeneca, RemeGen, NiKang Therapeutics, NGM Biopharmaceuticals, AstraZeneca, Adicet Bio, Allogene Therapeutics, Kura Oncology, Portage Biotech, HUTCHMED, Arsenal Biosciences, and others are evaluating new advanced kidney cancer drugs to improve the treatment landscape.
- Promising advanced kidney cancer pipeline therapies such as JANX008, IPI-549, Belzutifan, XmAbA 808, XL092, VB10.NEO, Savolitinib, RC198, NKT2152, NGM707, MEDI5752, ADI-270, ALLO 316, KO 2806, TT-10, Fruquintinib, AB 2100, and others are under different phases of advanced kidney cancer clinical trials.
- In April 2024, Arsenal Biosciences announced that the first patient had been dosed with AB-2100 in a multi-center, open-label Phase I/II clinical trial for patients with clear-cell renal cell carcinoma (ccRCC).
- In December 2023, Exelixis and Arcus Biosciences announced that the companies have entered into a clinical trial collaboration for STELLAR-009, a phase Ib/II trial evaluating zanzalintinib, Exelixis’ next-generation tyrosine kinase inhibitor (TKI), in combination with AB521, an inhibitor of the transcription factor HIF-2⍺, in patients with advanced solid tumors, including clear cell renal cell carcinoma (ccRCC).
- In September 2023, Merck announced the U.S. Food and Drug Administration (FDA) has accepted and granted priority review for a supplemental new drug application (sNDA) seeking approval for WELIREG, Merck’s oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, for the treatment of adult patients with advanced renal cell carcinoma (RCC) following immune checkpoint and anti-angiogenic therapies.
- In July 2023, China’s National Medical Products Administration (NMPA) accepted a supplemental new drug application (sNDA) seeking the approval of toripalimab (Tuoyi) plus axitinib (Inlyta) for the first-line treatment of patients with unresectable or metastatic renal cell carcinoma (RCC). The sNDA is supported by data from an interim analysis of the Phase III RENOTORCH trial (NCT04394975), which showed that the combination led to an improvement in progression-free survival (PFS) vs single-agent sunitinib (Sutent).
- In April 2023, Allogene Therapeutics presented interim data from its Phase I TRAVERSE trial of ALLO-316, the Company’s first AlloCAR T investigational product candidate for solid tumors, in an oral presentation at the American Association for Cancer Research (AACR) Annual Meeting in Orlando, Florida. The preliminary antitumor activity and encouraging safety profile of the novel anti-CD70 allogeneic CAR T-cell therapy ALLO-316 at low dose levels indicated that the regimen could represent a novel option for heavily pretreated patients with CD70-expressing advanced clear cell renal cell carcinoma (ccRCC).
- In March 2023, Telix announced presentations from the Company’s carbonic anhydrase IX (CAIX) targeting kidney and bladder cancer programs at the 38th Annual European Association of Urology (EAU) Congress which was held in Milan.
Request a sample and discover the recent advances in advanced kidney cancer treatment drugs @ Advanced Kidney Cancer Pipeline Report
The advanced kidney cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage advanced kidney cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the advanced kidney cancer clinical trial landscape.
Advanced Kidney Cancer Overview
Advanced kidney cancer, also referred to as advanced renal cell carcinoma, is the predominant form of kidney cancer, constituting over 90% of renal cancers in adults. Additional varieties include transitional cell carcinomas of the renal pelvis, which exhibit similar characteristics to bladder cancers, and renal sarcoma, a less common kidney tumor. The primary risk factor for renal cell carcinoma (RCC) is smoking tobacco products like cigarettes and cigars, while obesity, particularly among women, also poses a significant risk.
Administering systemic treatment for advanced or metastatic RCC necessitates oncologists well-versed in the condition, medication, side effects, drug interactions, and treatment supervision. Patients gain from a collaborative strategy involving nursing, dietary, and pharmacy assistance for optimal care. Consistent patient assessments are vital for promptly addressing and handling side effects, thus safeguarding patient well-being and treatment effectiveness. Furnishing thorough details on possible adverse reactions, preventive measures, and mitigation strategies to patients and caregivers is crucial for informed choices and caregiving.
Accurate staging plays a pivotal role in effectively managing renal cell carcinoma (RCC), as it dictates treatment options. For Stage 1a, where tumors are limited to the kidney, the preferred approach is complete surgical removal, ideally with partial nephrectomy to preserve kidney function. Surveillance entails abdominal CT or MRI within six months of treatment initiation, followed by yearly imaging. Stage 1b typically calls for partial or radical nephrectomy, with varying post-surgery imaging schedules. Stages 2 and 3 usually require radical nephrectomy, accompanied by specific imaging protocols for monitoring. In Stage 4, systemic targeted therapies such as VEGF-TKIs or rapamycin inhibitors are favored over immunotherapy, with nephrectomy followed by immunotherapy demonstrating improved survival rates. Follow-up involves pretreatment imaging and regular monitoring every 6 to 16 weeks, depending on the patient's clinical condition.
Find out more about advanced kidney cancer treatment drugs @ Drugs for Advanced Kidney Cancer Treatment
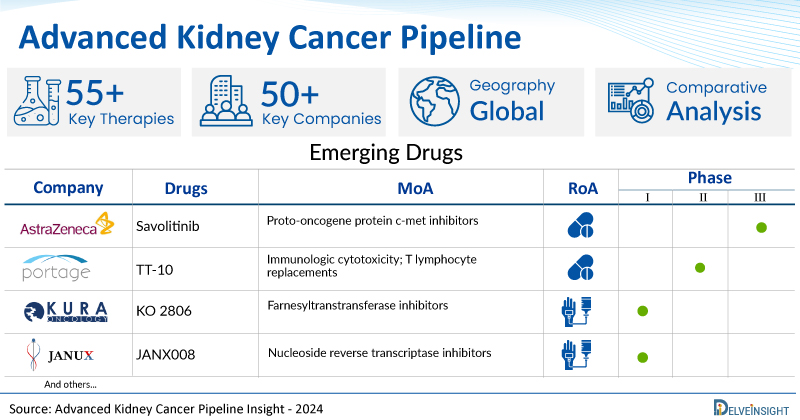
A snapshot of the Advanced Kidney Cancer Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Savolitinib | AstraZeneca | Phase III | Proto-oncogene protein c-met inhibitors | Oral |
| TT-10 | Portage Biotech | Phase I/II | Immunologic cytotoxicity; T lymphocyte replacements | Oral |
| KO 2806 | Kura Oncology | Phase I | Farnesyltranstransferase inhibitors | Oral |
| JANX008 | Janux Therapeutics | Phase I | Immunologic cytotoxicity; T lymphocyte stimulants | Intravenous |
| XmAb 808 | Xencor06 | Phase I | Antibody-dependent cell cytotoxicity; T lymphocyte stimulants | Intravenous |
| RC198 | RemeGen | Phase I | Interleukin 15 replacements | Subcutaneous |
Learn more about the emerging advanced kidney cancer pipeline therapies @ Advanced Kidney Cancer Clinical Trials
Advanced Kidney Cancer Therapeutics Assessment
The advanced kidney cancer pipeline report proffers an integral view of the advanced kidney cancer emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Advanced Kidney Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: Proto-oncogene protein c-met inhibitors, Antibody-dependent cell cytotoxicity, CD223 antigen inhibitors, T lymphocyte stimulants, Immunologic cytotoxicity, Axl receptor tyrosine kinase inhibitors, Immunomodulators, Protein tyrosine kinase inhibitors, Proto-oncogene protein c-mer inhibitors, Proto-oncogene protein c-met inhibitors, Vascular endothelial growth factor receptor antagonists, Interleukin 15 replacements
- Key Advanced Kidney Cancer Companies: Janux Therapeutics, Infinity Pharmaceuticals, Merck Sharp & Dohme, Xencor, Exelixis, Nykode Therapeutics, AstraZeneca, RemeGen, NiKang Therapeutics, NGM Biopharmaceuticals, AstraZeneca, Adicet Bio, Allogene Therapeutics, Kura Oncology, Portage Biotech, HUTCHMED, Arsenal Biosciences, and others
- Key Advanced Kidney Cancer Pipeline Therapies: JANX008, IPI-549, Belzutifan, XmAbA 808, XL092, VB10.NEO, Savolitinib, RC198, NKT2152, NGM707, MEDI5752, ADI-270, ALLO 316, KO 2806, TT-10, Fruquintinib,AB 2100, and others
Dive deep into rich insights for new drugs for advanced kidney cancer treatment, visit @ Advanced Kidney Cancer Drugs
Table of Contents
| 1. | Advanced Kidney Cancer Pipeline Report Introduction |
| 2. | Advanced Kidney Cancer Pipeline Report Executive Summary |
| 3. | Advanced Kidney Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Advanced Kidney Cancer Clinical Trial Therapeutics |
| 6. | Advanced Kidney Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Advanced Kidney Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Advanced Kidney Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Advanced Kidney Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Advanced Kidney Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Advanced Kidney Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Advanced Kidney Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the advanced kidney cancer pipeline therapeutics, reach out @ Advanced Kidney Cancer Treatment Drugs
Related Reports
Renal Cell Carcinoma Epidemiology Forecast
Renal Cell Carcinoma Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted renal cell carcinoma epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Renal Cell Carcinoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key renal cell carcinoma companies, including AstraZeneca, Infinity Pharmaceuticals, Ipsen, Novartis, Aveao pharmaceuticals, Merck Sharp & Dohme Corp., Pfizer, Bayer Healthcare, Incyte Corporation, GlaxoSmithKline, Bristol-Myers Squibb, Hoffman La Roche, among others.
Metastatic Renal Cell Carcinoma Market
Metastatic Renal Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic renal cell carcinoma companies including Nanobiotix, Aravive, Mirati Therapeutics Inc., BeiGene, Shanghai PerHum Therapeutics Co., Ltd., Akeso Biopharma, among others.
Renal Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key renal cell carcinoma companies including Exelixis, ColImmune, Akesobio, Corvus Pharmaceuticals, CRISPR Therapeutics, Allogene Therapeutics, Pfizer, Roche, AstraZeneca, Peloton Therapeutics, Inc., Arcus Biosciences, Inc., Merck Sharp & Dohme, Instil Bio, HUYABIO International, LLC., Xencor, Inc., MedImmune LLC, CRISPR Therapeutics, Merck Sharp & Dohme, Janux Therapeutics, Daiichi Sankyo, Inc., Calithera Biosciences, Inc, NiKang Therapeutics, Inc., Peloton Therapeutics, Inc., HiFiBiO Therapeutics, ProfoundBio US Co., HiberCell, Inc., Asher Biotherapeutics, Inc., Tarus Therapeutics, Inc., SOTIO Biotech AG, among others.
Metastatic Renal Cell Carcinoma Pipeline
Metastatic Renal Cell Carcinoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key metastatic renal cell carcinoma companies, including AstraZeneca, Genentech, Sumitomo Dainippon Pharma, Allogene Therapeutics, Nektar Therapeutics, Eli Lilly and Company, Xynomic Pharmaceuticals, AnewPharma, HUTCHMED, MedImmune, Incyte Corporation, NiKang Therapeutics, OncoC4, Inc., Nanobiotix, Aravive, Mirati Therapeutics Inc., BeiGene, Shanghai PerHum Therapeutics Co., Ltd., Akeso Biopharma, Novartis Pharmaceuticals, Vaccibody, SillaJen, Inc., Chongqing Precision Biotech Co., Ltd, Infinity Pharmaceuticals, Inc., SOTIO Biotech AG, Pfizer, Peloton Therapeutics, Inc., NeoTX Therapeutics Ltd., among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
