New York, USA, July 08, 2024 (GLOBE NEWSWIRE) -- Advanced Melanoma Clinical Trial Pipeline Analysis Demonstrates 55+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Advanced melanoma is when melanoma cells spread from where they started (the primary melanoma) to other parts of the body. The advanced melanoma therapeutics market is poised for significant growth, driven by the development of innovative biologics, particularly immunotherapy medications. These medications have demonstrated promising results in treating advanced melanoma, leading to increased adoption and market expansion. Furthermore, growing awareness of melanoma and its treatment options, as well as advancements in diagnostic techniques, are also contributing to the market's growth.
DelveInsight’s 'Advanced Melanoma Pipeline Insight 2024' report provides comprehensive global coverage of pipeline advanced melanoma therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the advanced melanoma pipeline domain.
Key Takeaways from the Advanced Melanoma Pipeline Report
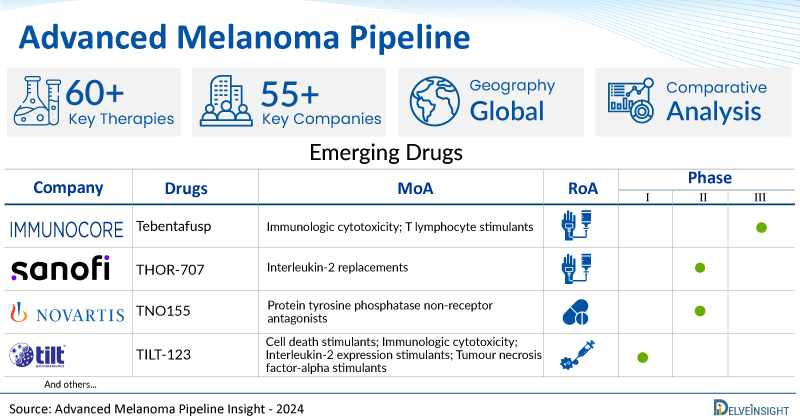
- DelveInsight’s advanced melanoma pipeline report depicts a robust space with 55+ active players working to develop 60+ pipeline therapies for advanced melanoma treatment.
- Key advanced melanoma companies such as Immunocore, Sanofi, TILT Biotherapeutics, Sumitomo Pharma, BioMed Valley Discoveries, Replimune, ImaginAb, Shanghai Pharmaceuticals, Seagen, Karyopharm Therapeutics, Hoffmann-La Roche, Qilu Pharmaceutical, PrimeVax Immuno-Oncology, Lyell Immunopharma, JS InnoPharm, Immunophotonics, Innovent Biologics (Suzhou), Bio-Thera Solutions, BeiGene, RemeGen, and others are evaluating new advanced melanoma drugs to improve the treatment landscape.
- Promising advanced melanoma pipeline therapies such as, Tebentafusp, THOR-707, TILT-123, TP-0903, Ulixertinib, Vusolimogene Oderparepvec, Zirconium Zr 89, T3011, SGN-BB228, Selinexor, RO6874281, QLF31907, PV-001-DC, LYL845, JSI-1187, IP-001, IBI363, BAT4706, BGB-A445, RC48-ADC, and others are under different phases of advanced melanoma clinical trials.
- In April 2024, Obsidian Therapeutics provided an update on its Phase I first-in-human study of OBX-115 tumor-infiltrating lymphocyte (TIL) cell therapy in patients with advanced or metastatic melanoma, including 25-week median study follow-up safety data and newly detailed efficacy data, during a presentation at the American Association for Cancer Research (AACR) Annual Meeting in San Diego, CA.
- In April 2024, Iovance Biotherapeutics announced that clinical data for lifileucel in combination with pembrolizumab in frontline advanced melanoma, as well as translational data, will be highlighted at the 2024 ASCO Annual Meeting.
- In December 2023, Obsidian Therapeutics announced positive top-line results from the ongoing first-in-human, Phase 1 clinical trial evaluating the safety and efficacy of OBX-115, Obsidian’s lead-engineered tumor-infiltrating lymphocyte (TIL) cell therapy candidate, in patients with Advanced or Metastatic Melanoma Post-Anti-PD1 Therapy.
- In November 2023, IO Biotech announced that it had completed enrollment in the pivotal Phase 3 clinical trial for IO102-IO103, in combination with KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, in patients with advanced melanoma.
- In November 2023, Lyell Immunopharma announced that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation to LYL845, an investigational tumor-infiltrating lymphocyte (TIL) product candidate for the treatment of patients with stage IIB-IV melanoma.
- In May 2023, Philogen S.p.A. and Sun Pharma announced the positive results from the Phase III PIVOTAL trial in patients with locally advanced fully resectable melanoma (NCT02938299).
Request a sample and discover the recent advances in advanced melanoma treatment drugs @ Advanced Melanoma Pipeline Report
The advanced melanoma pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage advanced melanoma drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the advanced melanoma clinical trial landscape.
Advanced Melanoma Overview
Advanced melanoma, a particularly challenging manifestation of skin cancer, presents significant complexities in treatment due to its tendency to metastasize or spread to distant parts of the body. This aggressive form of the disease, which often shows resistance to conventional treatments, has undergone a notable shift in treatment strategies with the introduction of immunotherapies, targeted therapies, vaccines, and combined treatments. Managing advanced melanoma involves strategies aimed at reducing or eliminating tumors, halting further spread, and easing symptoms. Various treatments such as surgery, radiation, immunotherapy, targeted therapy, and chemotherapy play pivotal roles in improving patient outcomes and their overall quality of life.
Melanoma arises from a combination of environmental, genetic, and immunological factors, with ongoing research focusing on leveraging the immune system for targeted therapies. Certain genes like CDKN2A, CDK4, MC1R, and the genetic disorder xeroderma pigmentosum, which affects the repair of UV-induced DNA damage, are linked to a predisposition to melanoma. Notably, there's a noticeable increase in the incidence of melanoma, particularly among white females.
Early-stage melanoma is typically addressed by surgically removing the tumor via wide excision or Mohs micrographic surgery, ensuring a definitive cure. Conversely, advanced melanoma, characterized by its spread to other parts of the body, poses considerable challenges for treatment due to its resistant nature and genetic diversity. The therapeutic landscape for metastatic melanoma has seen notable advancements, notably with the introduction of Vemurafenib (Zelboraf), which effectively targets the mutated BRAFV600E gene. However, despite initial success, patients often experience relapse within 8-12 months due to the reactivation of the MAPK pathway or other genetic mutations. While combining BRAF inhibitors with other MAPK inhibitors such as MEK or ERK can provide additional benefits, it also comes with an increased risk of toxicity. Despite these developments, there remains a gap in treatment options targeting RAS mutations. Although a KRASG12C inhibitor has demonstrated tumor regression in KRAS-mutant tumor models, its effectiveness against NRAS-mutated melanomas remains uncertain.
Find out more about advanced melanoma treatment drugs @ Drugs for Advanced Melanoma Treatment
A snapshot of the Advanced Melanoma Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Tebentafusp | Immunocore | Phase II/III | Immunologic cytotoxicity; T lymphocyte stimulants | Intravenous |
| THOR-707 | Sanofi | Phase II | Interleukin-2 replacements | Intravenous |
| TNO155 | Novartis | Phase II | Protein tyrosine phosphatase non-receptor antagonists | Oral |
| T3011 | Shanghai Pharmaceuticals Holding | Phase I/II | Antibody-dependent cell cytotoxicity; Cell death stimulants; Immunologic cytotoxicity; Interleukin-12 expression stimulants; Programmed cell death-1 receptor antagonists | Intratumoral |
| TILT-123 | TILT Biotherapeutics | Phase I | Cell death stimulants; Immunologic cytotoxicity; Interleukin-2 expression stimulants; Tumour necrosis factor-alpha stimulants | Intratumoral |
| TP-0903 | Sumitomo Pharma America | Phase I | Aurora kinase B inhibitors; Axl receptor tyrosine kinase inhibitors; Janus kinase-2 inhibitors; STAT6 transcription factor inhibitors | Oral |
Learn more about the emerging advanced melanoma pipeline therapies @ Advanced Melanoma Clinical Trials
Advanced Melanoma Therapeutics Assessment
The advanced melanoma pipeline report proffers an integral view of the advanced melanoma emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Advanced Melanoma Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: Immunologic cytotoxicity, T lymphocyte stimulants, Gene transference, Immunostimulants, Interleukin 12 replacements, Interleukin-2 replacements, Cell death stimulants, Interleukin-2 expression stimulants, Tumour necrosis factor-alpha stimulants, Protein tyrosine phosphatase non-receptor antagonists, Aurora kinase B inhibitors, Axl receptor tyrosine kinase inhibitors, Janus kinase-2 inhibitors, STAT6 transcription factor inhibitors
- Key Advanced Melanoma Companies: Immunocore, Sanofi, TILT Biotherapeutics, Sumitomo Pharma, BioMed Valley Discoveries, Replimune, ImaginAb, Shanghai Pharmaceuticals, Seagen, Karyopharm Therapeutics, Hoffmann-La Roche, Qilu Pharmaceutical, PrimeVax Immuno-Oncology, Lyell Immunopharma, JS InnoPharm, Immunophotonics, Innovent Biologics (Suzhou), Bio-Thera Solutions, BeiGene, RemeGen, and others
- Key Advanced Melanoma Pipeline Therapies: Tebentafusp, THOR-707, TILT-123, TP-0903, Ulixertinib, Vusolimogene Oderparepvec, Zirconium Zr 89, T3011, SGN-BB228, Selinexor, RO6874281, QLF31907, PV-001-DC, LYL845, JSI-1187, IP-001, IBI363, BAT4706, BGB-A445, RC48-ADC, and others
Dive deep into rich insights for new drugs for advanced melanoma treatment, visit @ Advanced Melanoma Drugs
Table of Contents
| 1. | Advanced Melanoma Pipeline Report Introduction |
| 2. | Advanced Melanoma Pipeline Report Executive Summary |
| 3. | Advanced Melanoma Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Advanced Melanoma Clinical Trial Therapeutics |
| 6. | Advanced Melanoma Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Advanced Melanoma Pipeline: Late-Stage Products (Phase III) |
| 8. | Advanced Melanoma Pipeline: Mid-Stage Products (Phase II) |
| 9. | Advanced Melanoma Pipeline: Early-Stage Products (Phase I) |
| 10. | Advanced Melanoma Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Advanced Melanoma Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Advanced Melanoma Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the advanced melanoma pipeline therapeutics, reach out @ Advanced Melanoma Treatment Drugs
Related Reports
Melanoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key melanoma companies, including BioMed Valley Discoveries, Inc, Amgen, Checkmate Pharmaceuticals, Astellas Pharma Inc, Galectin Therapeutics Inc., Eucure (Beijing) Biopharma Co., Ltd, Mologen AG, Ultimovacs ASA, Seagen Inc., MedImmune LLC, OnKure, Inc., among others.
Melanoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key melanoma companies including IO Biotech, Merck Sharp & Dohme, Eisai, Regeneron Pharmaceuticals, Iovance Biotherapeutics, Highlight Therapeutics, Linnaeus Therapeutics, Spring Bank Pharmaceuticals, Taiga Biotechnologies, Inc., Aivita Biomedical, Inc., Genentech, Inc., Dana-Farber Cancer Institute, BioMed Valley Discoveries, Inc, Amgen, Checkmate Pharmaceuticals, Astellas Pharma Inc, Galectin Therapeutics Inc., Eucure (Beijing) Biopharma Co., Ltd, Mologen AG, Ultimovacs ASA, Seagen Inc., MedImmune LLC, OnKure, Inc., Syntrix Biosystems, Inc., Anaveon AG, iOx Therapeutics, Portage Biotech, ModernaTX, Inc., Nektar Therapeutics, BioNTech SE, Sapience Therapeutics, HUYABIO International, LLC., Provectus Biopharmaceuticals, Inc., Cantargia AB, Turnstone Biologics, Corp., Aura Biosciences, F-star Therapeutics, Inc., Day One Biopharmaceuticals, Inc., Kinnate Biopharma, Xencor, Inc., HiFiBiO Therapeutics, among others.
Metastatic Melanoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic melanoma companies including Neon Therapeutics, BioNTech, Bristol-Myers Squibb, among others.
Refractory Metastatic Melanoma Pipeline
Refractory Metastatic Melanoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key refractory metastatic melanoma companies, including Idera Pharmaceuticals, BioNTech, Bristol-Myers Squibb, among others.
Refractory Metastatic Melanoma Market
Refractory Metastatic Melanoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key refractory metastatic melanoma companies including Idera Pharmaceuticals, BioNTech, Bristol-Myers Squibb, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
