New York, USA, Aug. 13, 2024 (GLOBE NEWSWIRE) -- Chronic Myelomonocytic Leukemia Clinical Trial Pipeline Analysis Demonstrates 22+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Chronic Myelomonocytic Leukemia (CMML) is a rare type of blood cancer characterized by features of both myelodysplastic syndromes and myeloproliferative neoplasms, primarily affecting older adults with a higher incidence in males. The market for CMML treatments is expected to see significant growth, driven by the increasing recognition of the disease and the development of novel therapies by pharmaceutical companies. This growth is further supported by ongoing clinical trials and research aimed at improving treatment outcomes, as CMML currently lacks disease-modifying therapies, highlighting a critical need for effective treatment options.
DelveInsight’s 'Chronic Myelomonocytic Leukemia Pipeline Insight 2024' report provides comprehensive global coverage of pipeline chronic myelomonocytic leukemia therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the chronic myelomonocytic leukemia pipeline domain.
Key Takeaways from the Chronic Myelomonocytic Leukemia Pipeline Report
- DelveInsight’s chronic myelomonocytic leukemia pipeline report depicts a robust space with 22+ active players working to develop 25+ pipeline therapies for chronic myelomonocytic leukemia treatment.
- Key chronic myelomonocytic leukemia companies such as Kura Oncology, Immune-Onc Therapeutics, Novartis Pharmaceuticals, Incyte Corporation, AbbVie, Nerviano Medical Sciences, Newave Pharmaceuticals, Amgen, and others are evaluating new chronic myelomonocytic leukemia drugs to improve the treatment landscape.
- Promising chronic myelomonocytic leukemia pipeline therapies such as Tipifarnib, IO-202, Sabatolimab, Ruxolitinib, Venetoclax, NMS-03592088, LP-108, AMG-176, and others are under different phases of chronic myelomonocytic leukemia clinical trials.
- In July 2024, the FDA placed a partial clinical hold on a phase I trial (NCT04734990) evaluating Seclidemstat (SP-2577) in combination with azacitidine (Vidaza) for the treatment of patients with myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML).
- In June 2024, Immune-Onc Therapeutics announced that the company will present additional positive interim Phase Ib expansion cohort data for IO-202 in patients with chronic myelomonocytic leukemia (CMML) at the 2024 European Hematology Association (EHA) Annual Meeting held virtually.
- In February 2024, Immune-Onc Therapeutics announced that the U.S. The Food and Drug Administration (FDA) granted Orphan Drug Designation for IO-202 for the treatment of chronic myelomonocytic leukemia (CMML).
- In January 2024, Taiho Oncology announced the publication of the final results from the pivotal ASCERTAIN clinical trial of fixed-dose oral decitabine and cedazuridine (INQOVI®) compared to intravenous decitabine in adults with intermediate and high-risk myelodysplastic syndromes (MDS) including chronic myelomonocytic leukemia (CMML).
- In April 2023, Nerviano Medical Sciences Srl, a member of NMS Group and a clinical-stage company discovering and developing innovative therapies for the treatment of cancer announced that data from the First-In-Human study of NMS-03592088, a novel, potent inhibitor of FLT3, KIT and CSF1R were presented during an oral scientific session at the American Association for Cancer Research 2023 Annual Meeting in Orlando, Florida. NMS-03592088 is currently being explored in MKIA-088-001 trial, a multi-center Phase I/II study to evaluate safety, tolerability, and efficacy in patients with relapsed or refractory (R/R) acute myeloid leukemia (AML) or chronic myelomonocytic leukemia (CMML).
Request a sample and discover the recent advances in chronic myelomonocytic leukemia treatment drugs @ Chronic Myelomonocytic Leukemia Pipeline Report
The chronic myelomonocytic leukemia pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage chronic myelomonocytic leukemia drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the chronic myelomonocytic leukemia clinical trial landscape.
Chronic Myelomonocytic Leukaemia Overview
Chronic myelomonocytic leukemia (CMML) is a clonal disorder of hematopoietic stem cells, exhibiting characteristics of both myelodysplastic syndromes and myeloproliferative neoplasms, with a 15-20% risk of transforming into acute leukemia over 3-5 years. This type of leukemia is marked by elevated levels of monocytes in the blood and bone marrow and is considered a rare blood cancer with mixed features of two other blood cancer types. Despite its name, the World Health Organization (WHO) classifies CMML as a ‘mixed myelodysplastic (MDS) myeloproliferative neoplasm (MPN)’. MPN disorders involve the bone marrow producing too many blood cells, whereas MDS affects the production of normal blood cells to varying degrees.
About half of CMML patients present with a high white blood cell count, resembling MPN, while the other half have normal or low white blood cell counts, making the disease more similar to MDS. Unlike chronic myeloid leukemia (CML), which affects a broad range of myeloid cells, CMML specifically impacts monocytes, which are crucial for fighting infections. Genetic mutations leading to CMML can result from aging, cytotoxic chemotherapy, or radiation exposure. CMML is not contagious. Diagnosis often follows a routine blood test showing abnormal monocytes, leading to further tests like additional blood work, bone marrow biopsy, or cytogenetic tests.
Find out more about chronic myelomonocytic leukemia treatment drugs @ Drugs for Chronic Myelomonocytic Leukemia Treatment
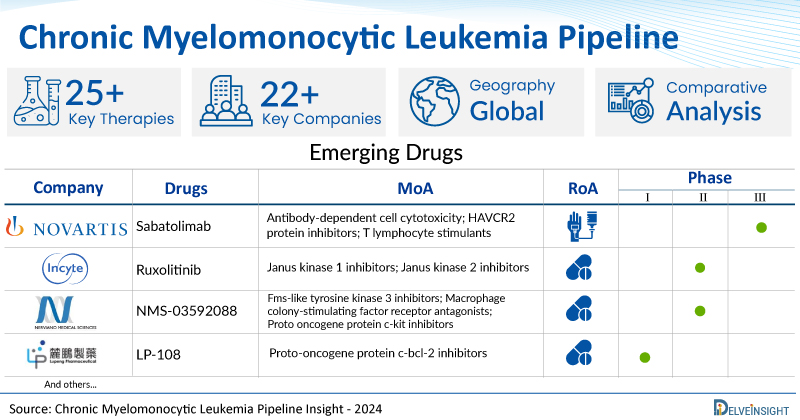
A snapshot of the Chronic Myelomonocytic Leukemia Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Sabatolimab | Novartis | Phase III | Antibody-dependent cell cytotoxicity; HAVCR2 protein inhibitors; T lymphocyte stimulants | Intravenous |
| Ruxolitinib | Incyte Corporation | Phase II | Janus kinase 1 inhibitors; Janus kinase 2 inhibitors | Oral |
| NMS-03592088 | Nerviano Medical Sciences | Phase I/II | Fms-like tyrosine kinase 3 inhibitors; Macrophage colony-stimulating factor receptor antagonists; Proto oncogene protein c-kit inhibitors | Oral |
| LP-108 | Guangzhou Lupeng Pharmaceutical | Phase I | Proto-oncogene protein c-bcl-2 inhibitors | Oral |
| Tapotoclax | Amgen | Phase I | MCL1 protein inhibitors | Intravenous |
Learn more about the emerging chronic myelomonocytic leukemia pipeline therapies @ Chronic Myelomonocytic Leukemia Clinical Trials
Chronic Myelomonocytic Leukaemia Therapeutics Assessment
The chronic myelomonocytic leukemia pipeline report proffers an integral view of the chronic myelomonocytic leukemia emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Chronic Myelomonocytic Leukemia Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: Antibody-dependent cell cytotoxicity, HAVCR2 protein inhibitors, T lymphocyte stimulants, Janus kinase 1 inhibitor, Janus kinase 2 inhibitors, Fms-like tyrosine kinase 3 inhibitors, Macrophage colony-stimulating factor receptor antagonists, Proto oncogene protein c-kit inhibitors, Proto-oncogene protein c-bcl-2 inhibitors, MCL1 protein inhibitors
- Key Chronic Myelomonocytic Leukemia Companies: Kura Oncology, Immune-Onc Therapeutics, Novartis Pharmaceuticals, Incyte Corporation, AbbVie, Nerviano Medical Sciences, Newave Pharmaceuticals, Amgen, and others
- Key Chronic Myelomonocytic Leukemia Pipeline Therapies: Tipifarnib, IO-202, Sabatolimab, Ruxolitinib, Venetoclax, NMS-03592088, LP-108, AMG-176, and others
Dive deep into rich insights for new drugs for chronic myelomonocytic leukemia treatment, visit @ Chronic Myelomonocytic Leukemia Drugs
Table of Contents
| 1. | Chronic Myelomonocytic Leukemia Pipeline Report Introduction |
| 2. | Chronic Myelomonocytic Leukemia Pipeline Report Executive Summary |
| 3. | Chronic Myelomonocytic Leukemia Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Chronic Myelomonocytic Leukemia Clinical Trial Therapeutics |
| 6. | Chronic Myelomonocytic Leukemia Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Chronic Myelomonocytic Leukemia Pipeline: Late-Stage Products (Phase III) |
| 8. | Chronic Myelomonocytic Leukemia Pipeline: Mid-Stage Products (Phase II) |
| 9. | Chronic Myelomonocytic Leukemia Pipeline: Early-Stage Products (Phase I) |
| 10. | Chronic Myelomonocytic Leukemia Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Chronic Myelomonocytic Leukemia Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Chronic Myelomonocytic Leukemia Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the chronic myelomonocytic leukemia pipeline therapeutics, reach out @ Chronic Myelomonocytic Leukemia Treatment Drugs
Related Reports
Chronic Myeloid Leukemia Epidemiology Forecast
Chronic Myeloid Leukemia Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted chronic myeloid leukemia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Chronic Myeloid Leukemia Pipeline
Chronic Myeloid Leukemia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic myeloid leukemia companies, including Kartos Therapeutics, Denovo Biopharma, Orbus Therapeutics, Onconeutics, among others.
Chronic Lymphocytic Leukemia Pipeline
Chronic Lymphocytic Leukemia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic lymphocytic leukemia companies, including Celgene, Loxo Oncology, Octapharma, among others.
Acute Myeloid Leukemia Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key acute myeloid leukemia companies, including Takeda Oncology, Immunity Bio, Teva Pharmaceuticals, among others.
Acute Myeloid Leukemia Pipeline
Acute Myeloid Leukemia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key acute myeloid leukemia companies, including Takeda Oncology, Immunity Bio, Teva Pharmaceuticals, among others.
Acute Lymphocytic Leukemia Market
Acute Lymphocytic Leukemia Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key acute lymphocytic leukemia companies, including AbbVie, Autolus Limited, Juventas Cell Therapy Ltd., Pinze Lifetechnology Co. Ltd., Celgene, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
