New York, USA, Oct. 30, 2024 (GLOBE NEWSWIRE) -- Advancements in Sensorineural Hearing Loss Clinical Trial Pipeline as 20+ Companies Pave the Way for Future Solutions | DelveInsight
Sensorineural hearing loss (SNHL) is the most common type of hearing loss, caused by damage to the inner ear (cochlea) or the auditory nerve pathways. Aging is the most significant risk factor for sensorineural hearing loss. As the global population ages, with more people entering the 60+ age group, the demand for hearing aids, cochlear implants, and other hearing loss treatments is growing rapidly.
DelveInsight’s 'Sensorineural Hearing Loss Pipeline Insight 2024' report provides comprehensive global coverage of pipeline sensorineural hearing loss therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the sensorineural hearing loss pipeline domain.
Key Takeaways from the Sensorineural Hearing Loss Pipeline Report
- DelveInsight’s sensorineural hearing loss pipeline report depicts a robust space with 20+ active players working to develop 20+ pipeline sensorineural hearing loss drugs.
- Key sensorineural hearing loss companies such as Sensorion, AudioCure Pharma, Reyon Pharmaceutical, Decibel Therapeutics, Sound Pharmaceuticals, Otonomy, Pipeline Therapeutics, Heyu (Suzhou) Pharmaceutical, Cilcare, and others are evaluating new sensorineural hearing loss drugs to improve the treatment landscape.
- Promising pipeline sensorineural hearing loss therapies such as SENS-401, AC-102, RY-103, DB-OTO, Ebselen, OTO 413, PIPE 505, HY-01, CIL-003, and others are under different phases of sensorineural hearing loss clinical trials.
- In August 2024, Ear Science Institute Australia’s project on sensorineural hearing loss treatment was selected by MedChem Australia to be included in the first round of Portfolio and Pilot projects that will receive support with the provision of medicinal chemistry and pharmacokinetics expertise for promising drug discovery programs tackling a range of diseases.
- In August 2024, Sound Pharmaceuticals wished to maintain its relationship with WuXi AppTec for manufacturing its lead Meniere’s disease drug SPI-1005, amidst increasing scrutiny on Chinese companies in the US.
- In July 2024, Astellas Pharma announced that it had entered into an agreement with Frequency Therapeutics to co-develop and commercialize FX-322, a regenerative therapy to treat hearing loss.
- In July 2024, Sound Pharmaceuticals completed a Phase III trial for its drug SPI-1005. Interim results from the study are expected this quarter. The STOPMD-3 trial investigated the effects of oral SPI-1005 in 201 Meniere’s disease patients to restore sensory neural hearing loss.
- In July 2024, Sensorion SA announced that the company has reported new efficacy endpoints data from SENS-401 in a Phase II study for the preservation of residual hearing loss.SENS-401, a 5HT3R antagonist that blocks the CalN pathway, reduced sudden hearing loss as measured by the change of hearing threshold from baseline to the end of the treatment period in the implanted ear at several frequencies.
- In January 2024, Akouos, Inc., a wholly owned subsidiary of Eli Lilly and Company announced positive initial clinical results from the Phase I/II AK-OTOF-101 study, which demonstrated pharmacologic hearing restoration within 30 days of AK-OTOF administration in the first participant, an individual with a decade-plus history of profound hearing loss.
Request a sample and discover the recent advances in sensorineural hearing loss drugs @ Sensorineural Hearing Loss Pipeline Report
The sensorineural hearing loss pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage sensorineural hearing loss drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the sensorineural hearing loss clinical trial landscape.
Sensorineural Hearing Loss Overview
Sensorineural hearing loss (SHL) occurs due to damage to the structures within the inner ear or the auditory nerve, accounting for over 90% of hearing loss in adults. Common factors leading to SNHL include exposure to loud sounds, genetic predispositions, and the natural aging process. People with SHL may struggle with understanding speech, hearing soft sounds, or differentiating between various sounds. Diagnosis usually requires a thorough hearing assessment conducted by an audiologist, which may encompass a physical exam, hearing tests, and imaging procedures.
SHL presents a range of signs and symptoms that can affect a person's auditory perception. Common signs include difficulty in comprehending speech, hearing sounds as muffled, a feeling of fullness in the ears, and a gradual or sudden hearing loss. Those with SHL may find it hard to engage in conversations in noisy settings, experience tinnitus, have trouble distinguishing high-pitched sounds, and perceive speech as unclear or slurred.
Currently, there are no surgical treatments for SNHL. The primary options available are hearing aids and cochlear implants, which assist in managing hearing loss. Gene therapy for hearing loss is a developing area of research, but it has not yet been implemented in clinical settings for SNHL.
Find out more about sensorineural hearing loss drugs @ Sensorineural Hearing Loss Analysis
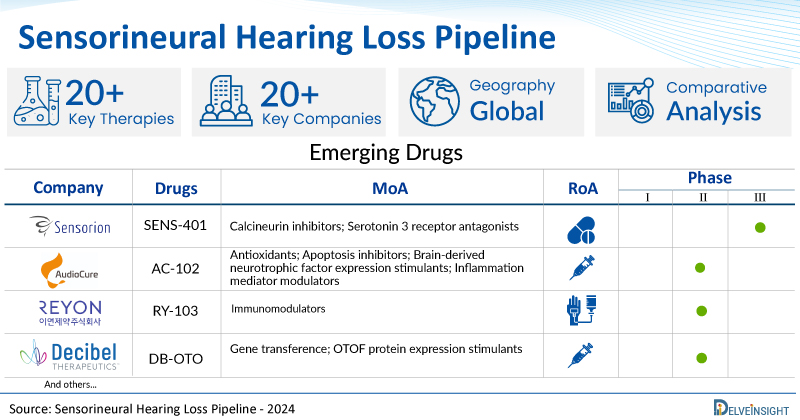
A snapshot of the Pipeline Sensorineural Hearing Loss Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| SENS-401 | Sensorion | II/III | Calcineurin inhibitors; Serotonin 3 receptor antagonists | Oral |
| AC-102 | AudioCure Pharma | II | Antioxidants; Apoptosis inhibitors; Brain-derived neurotrophic factor expression stimulants; Inflammation mediator modulators | Intratympanic injection |
| Ebselen | Sound Pharmaceuticals | II | Antioxidants; Cyclo-oxygenase 2 inhibitors; Glutathione peroxidase stimulants; Leukotriene B4 receptor antagonists; Prostaglandin receptor antagonists; Viral papain-like protease inhibitors; Viral protein inhibitors; Virus replication inhibitors | Oral |
| RY-103 | Reyon Pharmaceutical | I/II | Immunomodulators | Intravenous |
| DB-OTO | Decibel Therapeutics | I/II | Gene transference; OTOF protein expression stimulants | Intracochlear injection |
| CIL-003 | Cilcare | Preclinical | Undefined mechanism | Not disclosed |
Learn more about the emerging sensorineural hearing loss therapies @ Sensorineural Hearing Loss Clinical Trials
Sensorineural Hearing Loss Therapeutics Assessment
The sensorineural hearing loss pipeline report proffers an integral view of the emerging sensorineural hearing loss therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Sensorineural Hearing Loss Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: Calcineurin inhibitors, Serotonin 3 receptor antagonists, Apoptosis inhibitors, Brain-derived neurotrophic factor expression stimulants, Inflammation mediator modulators, Antioxidants, Cyclo-oxygenase 2 inhibitors, Glutathione peroxidase stimulants, Leukotriene B4 receptor antagonists, Prostaglandin receptor antagonists, Viral papain-like protease inhibitors, Viral protein inhibitors, Virus replication inhibitors, Amyloid precursor protein secretase inhibitors, Notch signaling pathway inhibitors, Immunomodulators, Gene transference, OTOF protein expression stimulants
- Key Sensorineural Hearing Loss Companies: Sensorion, AudioCure Pharma, Reyon Pharmaceutical, Decibel Therapeutics, Sound Pharmaceuticals, Otonomy, Pipeline Therapeutics, Heyu (Suzhou) Pharmaceutical, Cilcare, and others.
- Key Sensorineural Hearing Loss Pipeline Therapies: SENS-401, AC-102, RY-103, DB-OTO, Ebselen, OTO 413, PIPE 505, HY-01, CIL-003, and others.
Dive deep into rich insights for new sensorineural hearing loss treatments, visit @ Sensorineural Hearing Loss Drugs
Table of Contents
| 1. | Sensorineural Hearing Loss Pipeline Report Introduction |
| 2. | Sensorineural Hearing Loss Pipeline Report Executive Summary |
| 3. | Sensorineural Hearing Loss Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Sensorineural Hearing Loss Clinical Trial Therapeutics |
| 6. | Sensorineural Hearing Loss Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Sensorineural Hearing Loss Pipeline: Late-Stage Products (Phase III) |
| 8. | Sensorineural Hearing Loss Pipeline: Mid-Stage Products (Phase II) |
| 9. | Sensorineural Hearing Loss Pipeline: Early-Stage Products (Phase I) |
| 10. | Sensorineural Hearing Loss Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Sensorineural Hearing Loss Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Sensorineural Hearing Loss Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the sensorineural hearing loss pipeline therapeutics, reach out @ Sensorineural Hearing Loss Therapeutics
Related Reports
Sensorineural Hearing Loss Market
Sensorineural Hearing Loss Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key sensorineural hearing loss companies, including kouos Inc., Eli Lilly and Company, Frequency Therapeutics, Sensorion, Pipeline Therapeutics Inc., Cochlear, Erchonia Corporation, Otonomy Inc., Envoy Medical Corporation, Earlogic Korea Inc., Auris Medical Inc., among others.
Sudden Sensorineural Hearing Loss Market
Sudden Sensorineural Hearing Loss Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key sudden sensorineural hearing companies including Astellas Pharma, Cell Mogrify, Pfizer, Decibel Therapeutics, Sound Pharmaceuticals, among others.
Sudden Sensorineural Hearing Loss Pipeline
Sudden Sensorineural Hearing Loss Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key sudden sensorineural hearing loss companies, including Astellas Pharma, Cell Mogrify, Pfizer, Decibel Therapeutics, Sound Pharmaceuticals, among others.
Hearing Loss Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hearing loss companies, including Acousia Therapeutics, Decibel Therapeutics, Otonomy Inc., Sensorion, Autifony Therapeutics, Auris Medical, Sound Pharmaceuticals, Anida Pharma Inc., Gateway Biotechnology, Myrtelle Inc., Lineage Cell Therapeutics, Inc., Altamira Therapeutics, Hoba Therapeutics, Rinri Therapeutics, Autifony Therapeutics, Otologic Pharmaceutics, Audion Therapeutics, Perha Pharmaceuticals, Applied Genetic Technologies Corporation, Akouos, Inc., Oricula Therapeutics, Spiral Therapeutics, Pipeline Therapeutics, Prime Medicine, Boehringer Ingelheim, Autigen, Heyu (Suzhou) Pharmaceutical Technology Co., Ltd, Astellas Pharma, Mogrify Limited, among others.
Hearing Aid Devices Market Insight, Competitive Landscape, and Market Forecast – 2030 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key hearing aid devices companies involved, such as Amplifon, Audina Hearing Instruments, Inc., Sonova, Demant A/S, audifon GmbH & Co. KG, GN Store Nord A/S, Arphi Electronics Private Limited, Foshan Vohom Technology Co., Ltd., RION Co., Ltd., Eargo Inc, Elkon Pvt Ltd., Starkey Laboratories, Inc., WS Audiology Denmark A/S, Horentek Hearing Diagnostics, Bernafon, Unitron, MDHearingAid, Istok Audio Trading LLC, AlgorKorea Co., Ltd., Microson, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
