New York, USA, Nov. 20, 2024 (GLOBE NEWSWIRE) -- Hidradenitis Suppurativa Market to Showcase Remarkable Growth at a CAGR of 12.4% by 2034 | DelveInsight
According to DelveInsight’s analysis, the growth of the hidradenitis suppurativa market is expected to be mainly driven by the rise in awareness and access to treatment, recent approval of biologic therapies, anticipated approval of JAK-inhibitors & nanobodies and robust pipeline activity in the 7MM.
DelveInsight’s Hidradenitis Suppurativa Market Insights report includes a comprehensive understanding of current treatment practices, emerging hidradenitis suppurativa drugs, market share of individual therapies, and current and forecasted hidradenitis suppurativa market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Hidradenitis Suppurativa Market Report
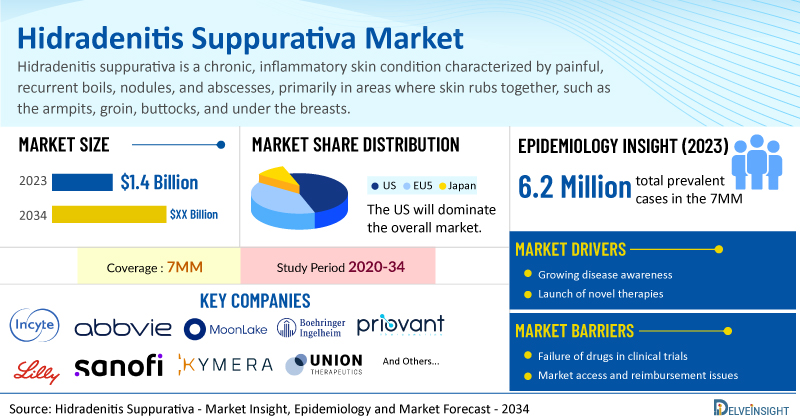
- Hidradenitis suppurativa market is currently underserved. It is anticipated that similar to rheumatoid arthritis, hidradenitis suppurativa has a sufficiently large market potential to support the co-existence of several blockbuster medications. According to DelveInsight’s analysis, the market size of hidradenitis suppurativa in the 7MM was USD 1.4 billion in 2023 and it is expected to increase by 2034.
- The total prevalent cases of hidradenitis suppurativa in the 7MM were approximately 6.2 million cases in 2023.
- Prominent companies working in the domain of hidradenitis suppurativa, including Incyte Corporation, AbbVie, MoonLake Immunotherapeutics, Boehringer Ingelheim, Priovant Therapeutics, Eli Lilly, Incyte Corporation, Sanofi, Kymera Therapeutics, UNION Therapeutics, and others, are actively working on innovative hidradenitis suppurativa drugs. These novel therapies are anticipated to enter the hidradenitis suppurativa market in the forecast period and are expected to change the market.
- Some of the key hidradenitis suppurativa treatments include Povorcitinib (INCB054707), RINVOQ (upadacitinib), Lutikizumab (ABT-981), Sonelokimab (M1095), Spesolimab, Brepocitinib (PF-06700841), Eltrekibart (LY3041658), Ruxolitinib 1.5% Cream, SAR-444656 (KT-474), Orismilast, and others.
- In August 2024, ACELYRIN announced plans to complete ongoing trials of Izokibep and suspend new investment in hidradenitis suppurativa and psoriatic arthritis.
- In June 2024, UCB’s BIMZELX (bimekizumab) was approved by the UK's Medicines and Healthcare products Regulatory Agency (MHRA) to treat adults with active moderate-to-severe hidradenitis suppurativa.
- In May 2024, MoonLake Immunotherapeutics announced that the first patients have been screened at a US trial site in its global Phase III clinical program, VELA, evaluating sonelokimab, an investigational Nanobody designed for moderate-to-severe hidradenitis suppurativa.
- In April 2024, the FDA accepted for review the supplemental Biologics License Application (sBLA) for BIMZELX for the treatment of adults with moderate-to-severe hidradenitis suppurativa. In addition, a second sBLA for the BIMZELX 2mL device presentations has also been accepted. Even after delivering promising data for BIMZELX hasn’t yet been approved in the US for hidradenitis suppurativa.
- In March 2024, OPZELURA in the Phase II trial met its primary endpoint, demonstrating a significantly greater reduction in abscess and inflammatory nodule (AN) count in patients treated with ruxolitinib cream 1.5%, compared to those who applied the vehicle control at Week 16 presented in American Academy of Dermatology (AAD) Annual Meeting.
Discover which therapies are expected to grab the hidradenitis suppurativa market share @ Hidradenitis Suppurativa Market Report
Hidradenitis Suppurativa Overview
Hidradenitis suppurativa is a chronic, inflammatory skin condition characterized by painful, recurrent boils, nodules, and abscesses, primarily in areas where skin rubs together, such as the armpits, groin, buttocks, and under the breasts. These lesions can often lead to tunneling of the skin and scarring over time.
The exact cause of hidradenitis suppurativa is not fully understood, but it is thought to involve a combination of genetic, hormonal, and environmental factors. Inflammation begins in the hair follicles, leading to blockages and subsequent rupture, which causes painful lesions. Risk factors include obesity, smoking, a family history of hidradenitis suppurativa, and certain hormonal changes, such as those related to the menstrual cycle.
Symptoms of hidradenitis suppurativa vary from mild to severe and may include painful bumps that enlarge and break open, leaking fluid, or pus with an unpleasant odor. Over time, these lesions can heal, leaving thick scars. The chronic nature of the disease, with its frequent flare-ups and the potential for severe pain and scarring, often leads to significant physical and emotional distress.
Diagnosis of hidradenitis suppurativa is primarily clinical, based on the appearance of the skin lesions and the characteristic pattern of their recurrence. Dermatologists often rely on a patient’s medical history, physical examination, and sometimes imaging studies like ultrasound to assess the extent of the disease. Early diagnosis and treatment are crucial to managing symptoms, minimizing complications, and improving the quality of life for those affected by hidradenitis suppurativa.
Hidradenitis Suppurativa Epidemiology Segmentation
The hidradenitis suppurativa epidemiology section provides insights into the historical and current hidradenitis suppurativa patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The hidradenitis suppurativa market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Prevalent Cases of Hidradenitis Suppurativa
- Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa
- Gender-specific Cases of Hidradenitis Suppurativa
- Age-specific Cases of Hidradenitis Suppurativa
- Stage-specific Cases of Hidradenitis Suppurativa
- Treated Cases of Hidradenitis Suppurativa
Download the report to understand which factors are driving hidradenitis suppurativa epidemiology trends @ Hidradenitis Suppurativa Epidemiological Insights
Hidradenitis Suppurativa Treatment Market
Since hidradenitis suppurativa has no cure, early diagnosis and treatment are crucial in preventing the disease from worsening and minimizing scarring. The treatment approach varies based on the severity and clinical stage of the disease. Available pharmacological treatments include topical and systemic antibiotics, corticosteroids, hormonal therapy, systemic retinoids, zinc supplements, and immunosuppressive agents like biologics. For mild cases, treatment typically involves antibacterial washes and topical antibiotics, while acute flare-ups may be managed with intralesional corticosteroids and minor surgical procedures.
Oral therapies for mild-to-moderate cases often involve extended courses of broad-spectrum antibiotics and systemic retinoids. Off-label treatments commonly use antibiotics such as clindamycin, rifampicin, and tetracycline due to their proven efficacy in studies.
In the current hidradenitis suppurativa treatment market, established therapies include BIMZELX (bimekizumab), COSENTYX (secukinumab), and HUMIRA (adalimumab). HUMIRA dominated the market until 2023, despite its US patent expiring in December 2016; AbbVie's stronger patents in the US provided additional protection for the drug's exclusivity.
However, in June 2023, the European Commission approved COSENTYX, an IL-17A monoclonal antibody, for adults with active moderate-to-severe hidradenitis suppurativa who have not responded adequately to conventional systemic therapy. The FDA also approved COSENTYX for the same indication in October 2023. More recently, in April 2024, UCB announced that the European Commission granted marketing authorization for BIMZELX to treat adults with active moderate-to-severe hidradenitis suppurativa who have not responded adequately to conventional systemic therapy.
Even though COSENTYX joined the market about ten years after HUMIRA, it has already established itself as a major player in hidradenitis suppurativa treatment space. According to reports, COSENTYX is experiencing a strong launch momentum. Although BIMZELX is more effective, COSENTYX has the first-mover advantage. It is worth highlighting that BIMZELX's efficacy is differentiated to both HUMIRA and COSENTYX. Although Novartis' early approval has allowed COSENTYX some leeway in treating hidradenitis suppurativa, UCB's BIMZELX, that yielded "incrementally better" outcomes than COSENTYX, might pose a serious threat to the medication in the near future.
Learn more about the market of hidradenitis suppurativa @ Hidradenitis Suppurativa Treatment
Hidradenitis Suppurativa Emerging Drugs and Companies
Some of the drugs in the pipeline include Eltrekibart (Eli Lilly), Sonelokimab (MoonLake Immunotherapeutics), Brepocitinib (Priovant Therapeutics), Spesolimab (Boehringer Ingelheim), Orismilast (UNION Therapeutics), Povorcitinib (Incyte Corporation), and others.
Eli Lilly is researching LY3041658, a monoclonal antibody targeting CXCR1/2 ligands. LY3041658 is capable of inhibiting ELR+CXC chemokine-induced Ca2+ mobilization, CXCR2 internalization, and chemotaxis in vitro, as well as neutrophil mobilization in vivo, without impairing other neutrophil functions. Along with demonstrating activity in both in vitro and in vivo settings, the epitope and structural basis of LY3041658's binding were analyzed using alanine scanning, crystallography, and mutagenesis. The trial began in the fourth quarter of 2020 and has progressed to Phase II development. This Phase II trial is a multicenter, randomized, double-blind, placebo-controlled study aimed at assessing the efficacy and safety of LY3041658 in adults with moderate-to-severe hidradenitis suppurativa.
Povorcitinib, developed by Incyte Corporation, is a selective JAK1 inhibitor that successfully reduces abscesses and inflammatory nodules in patients with moderate-to-severe hidradenitis suppurativa. Phase III data is anticipated in 2025, with a potential launch for treating hidradenitis suppurativa expected between 2026 and 2027.
The other pipeline therapies for hidradenitis suppurativa include
- RINVOQ: AbbVie
- Lutikizumab: AbbVie
- Ruxolitinib 1.5% Cream: Incyte Corporation
- Sonelokimab: MoonLake Immunotherapeutics
- Spesolimab: Boehringer Ingelheim
- SAR-444656/KT-474: Sanofi/Kymera Therapeutics
The anticipated launch of these emerging therapies are poised to transform the hidradenitis suppurativa market landscape in the coming years.
- The Janus kinase inhibitors (JAKi), which may not be available in the US market for 2–3 years, would provide patients with their first orally administered agent.
- Since nanobodies may target many pathways and have superior tissue penetration, there is lots of enthusiasm for nanobodies about their potential to treat hidradenitis suppurativa. The potential for Sonelokimab to replace COSENTYX exists if Moonlake's Phase II findings are similarly replicated in Phase III.
As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the hidradenitis suppurativa market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about hidradenitis suppurativa clinical trials, visit @ Hidradenitis Suppurativa Treatment Drugs
Hidradenitis Suppurativa Market Dynamics
The hidradenitis suppurativa market dynamics are anticipated to change in the coming years. The hidradenitis suppurativa market is entering an unprecedented era of rapid growth, driven by a surge in diverse pipeline therapeutics targeting Interleukins (i.e., IL-17, IL-36), anti-TNF, JAK inhibitors, and anti-complement factors.
Promising treatment options, including anti-IL-17 and anti-IL-1α inhibition, may open new doors for patients who do not respond to currently available therapeutics, particularly in ADA-refractory moderate to severe cases. Global collaborations will be vital in addressing the existing challenges in both mechanistic research and clinical management of hidradenitis suppurativa.
Furthermore, many potential therapies are being investigated for the treatment of hidradenitis suppurativa, and it is safe to predict that the treatment space will significantly impact the hidradenitis suppurativa market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the hidradenitis suppurativa market in the 7MM.
However, several factors may impede the growth of the hidradenitis suppurativa market. Despite the absence of definite biomarkers for early and progressive forms of the disease, substantial delays in diagnosis—averaging between 7 and 10 years—persist, largely due to low disease awareness, associated misdiagnoses, and under-reporting by patients because of shame and embarrassment.
Additionally, the market value of HUMIRA in Europe has been declining since 2018, with a faster decline expected due to the launch of multiple biosimilars in 2019 and the upcoming years. Many therapies have recently failed in clinical trials or were terminated due to safety issues.
Moreover, hidradenitis suppurativa treatment poses a significant economic burden and disrupts patients’ overall well-being and Quality of life (QoL). Furthermore, the hidradenitis suppurativa market growth may be offset by unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists.
| Hidradenitis Suppurativa Report Metrics | Details |
| Study Period | 2020–2034 |
| Hidradenitis Suppurativa Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Hidradenitis Suppurativa Market CAGR | 12.4% |
| Hidradenitis Suppurativa Market Size in 2023 | USD 1.4 Billion |
| Key Hidradenitis Suppurativa Companies | Incyte Corporation, AbbVie, MoonLake Immunotherapeutics, Boehringer Ingelheim, Priovant Therapeutics, Eli Lilly, Incyte Corporation, Sanofi, Kymera Therapeutics, UNION Therapeutics, and others |
| Key Hidradenitis Suppurativa | Povorcitinib (INCB054707), RINVOQ (upadacitinib), Lutikizumab (ABT-981), Sonelokimab (M1095), Spesolimab, Brepocitinib (PF-06700841), Eltrekibart (LY3041658), Ruxolitinib 1.5% Cream, SAR-444656/KT-474, Orismilast, and others |
Scope of the Hidradenitis Suppurativa Market Report
- Hidradenitis Suppurativa Therapeutic Assessment: Hidradenitis Suppurativa current marketed and emerging therapies
- Hidradenitis Suppurativa Market Dynamics: Conjoint Analysis of Emerging Hidradenitis Suppurativa Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Hidradenitis Suppurativa Market Access and Reimbursement
Discover more about hidradenitis suppurativa in development @ Hidradenitis Suppurativa Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | Hidradenitis Suppurativa Market Overview at a Glance: 7MM |
| 3.1 | Market Share by Therapies (%) Distribution of Hidradenitis Suppurativa in 2020 |
| 3.2 | Market Share by Therapies (%) Distribution of Hidradenitis Suppurativa in 2034 |
| 4 | Executive Summary of Hidradenitis Suppurativa |
| 5 | Key Events |
| 6 | Epidemiology and Market Methodology |
| 7 | Disease Overview: Hidradenitis Suppurativa |
| 7.1 | Introduction |
| 7.2 | Causes and Symptoms of Hidradenitis Suppurativa |
| 7.3 | Classification and Severity Assessment of Hidradenitis Suppurativa |
| 7.3.1 | Hurley Stages of hidradenitis suppurativa |
| 7.3.2 | Modified Sartorius Score (MSS) of hidradenitis suppurativa |
| 7.3.3 | Physician Global Assessment of Hidradenitis Suppurativa |
| 7.3.4 | Specific Severity Index of Hidradenitis Suppurativa (HSSI) |
| 7.4 | Pathophysiology of Hidradenitis Suppurativa |
| 7.5 | Genetic Basis of Hidradenitis Suppurativa |
| 7.6 | Diagnosis of Hidradenitis Suppurativa |
| 7.6.1 | Differential Diagnosis |
| 7.7 | Biomarkers of Hidradenitis Suppurativa |
| 7.8 | Risk Factors and Complications |
| 7.9 | Comorbidities |
| 8 | Treatment and Guidelines |
| 8.1 | S2k Guideline for the Treatment of Hidradenitis Suppurativa/ Acne Inversa (2024): Germany |
| 8.2 | European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa: (2015) |
| 8.3 | North American Clinical Management Guidelines for Hidradenitis Suppurativa: (2019) |
| 8.4 | British Association of Dermatologists Guidelines for the Management of Hidradenitis Suppurativa (Acne Inversa): (2018) |
| 8.5 | Guidelines for the Management of Hidradenitis Suppurativa: Recommendations Supported by the Centre of Evidence of the French Society of Dermatology: (2021) |
| 8.6 | Hidradenitis Suppurativa/Acne Inversa: A Practical Framework for Treatment Optimization – Systematic Review and Recommendations from the Hidradenitis Suppurativa Alliance Working Group: (2018) |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale: 7MM |
| 9.3 | Total Prevalent Cases of Hidradenitis Suppurativa in the 7MM |
| 9.4 | Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa in the 7MM |
| 9.5 | The United States |
| 9.5.1 | Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa in the US |
| 9.5.2 | Gender-specific Prevalent Cases of Hidradenitis Suppurativa in the US |
| 9.5.3 | Age-specific Prevalent Cases of Hidradenitis Suppurativa in the US |
| 9.5.4 | Stage-specific Prevalent Cases of Hidradenitis Suppurativa in the US |
| 9.5.5 | Total Treated Prevalent Cases of Hidradenitis Suppurativa in the US |
| 9.6 | EU4 and the UK |
| 9.6.1 | Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa in EU4 and the UK |
| 9.6.2 | Gender-specific Prevalent Cases of Hidradenitis Suppurativa in EU4 and the UK |
| 9.6.3 | Age-specific Prevalent Cases of Hidradenitis Suppurativa in EU4 and the UK |
| 9.6.4 | Stage-specific Prevalent Cases of Hidradenitis Suppurativa in EU4 and the UK |
| 9.6.5 | Total Treated Prevalent Cases of Hidradenitis Suppurativa in EU4 and the UK |
| 9.7 | Japan |
| 9.7.1 | Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa in Japan |
| 9.7.2 | Gender-specific Prevalent Cases of Hidradenitis Suppurativa in Japan |
| 9.7.3 | Age-specific Prevalent Cases of Hidradenitis Suppurativa in Japan |
| 9.7.4 | Stage-specific Prevalent Cases of Hidradenitis Suppurativa in Japan |
| 9.7.5 | Total Treated Prevalent Cases of Hidradenitis Suppurativa in Japan |
| 10 | Patient Journey of Hidradenitis Suppurativa |
| 11 | Key Endpoints in Hidradenitis Suppurativa Clinical Trials |
| 12 | Marketed Drugs |
| 12.1 | Key Competitors |
| 12.2 | HUMIRA (adalimumab): AbbVie/Eisai |
| 12.2.1 | Product Description |
| 12.2.2 | Regulatory milestones |
| 12.2.3 | Other Developmental Activities |
| 12.2.4 | Safety and Efficacy |
| 12.3 | COSENTYX (secukinumab): Novartis |
| 12.3.1 | Product Description |
| 12.3.2 | Regulatory Milestone |
| 12.3.3 | Other Developmental Activities |
| 12.3.4 | Clinical Development |
| 1.1.1.1 | Clinical Trials Information |
| 12.3.5 | Safety and Efficacy |
| 12.5 | BIMZELX (bimekizumab): UCB Biopharma |
| 12.5.1 | Product description |
| 12.5.2 | Regulatory Milestone |
| 12.5.3 | Other developmental Activities |
| 12.5.4 | Clinical Development |
| 12.5.4.1 | Clinical Trials Information |
| 12.5.5 | Safety and efficacy |
| 13 | Emerging Drugs |
| 13.1 | Key Cross |
| 13.2 | Povorcitinib (INCB054707): Incyte Corporation |
| 13.2.1 | Product Description |
| 13.2.2 | Other Developmental Activities |
| 13.2.3 | Clinical Development |
| 13.2.3.1 | Clinical Trials Information |
| 13.2.4 | Safety and Efficacy |
| 13.3 | Izokibep: ACELYRIN |
| 13.3.1 | Product Description |
| 13.3.2 | Other Developmental Activities |
| 13.3.3 | Clinical Development |
| 13.3.3.1 | Clinical Trials Information |
| 13.3.4 | Safety and Efficacy |
| 13.4 | RINVOQ (upadacitinib): AbbVie |
| 13.4.1 | Product Description |
| 13.4.2 | Other Developmental Activities |
| 13.4.3 | Clinical Development |
| 13.4.3.1 | Clinical Trials Information |
| 13.4.4 | Safety and Efficacy |
| 13.5 | Lutikizumab (ABT-981): AbbVie |
| 13.5.1 | Product description |
| 13.5.2 | Clinical Development |
| 13.5.2.1 | Clinical Trial Information |
| 13.5.3 | Safety and Efficacy |
| 13.6 | Sonelokimab (M1095): MoonLake Immunotherapeutics |
| 13.6.1 | Product Description |
| 13.6.2 | Other Developmental Activity |
| 13.6.3 | Clinical Development |
| 13.6.3.1 | Clinical Trial Information |
| 13.6.4 | Safety and Efficacy |
| 13.7 | Spesolimab: Boehringer Ingelheim |
| 13.7.1 | Product Description |
| 13.7.2 | Clinical Development |
| 13.7.2.1 | Clinical Trials Information |
| 13.7.3 | Safety and Efficacy |
| 13.8 | Brepocitinib (PF-06700841): Priovant Therapeutics |
| 13.8.1 | Product Description |
| 13.8.2 | Clinical Development |
| 13.8.2.1 | Clinical Trial Information |
| 13.8.3 | Safety and Efficacy |
| 13.9 | Eltrekibart (LY3041658): Eli Lilly |
| 13.9.1 | Product Description |
| 13.9.2 | Clinical Development |
| 13.9.2.1 | Clinical Trial Information |
| 13.9.3 | Safety and Efficacy |
| 13.1 | Ruxolitinib 1.5% Cream: Incyte Corporation |
| 13.10.1 | Product Description |
| 13.10.2 | Other Developmental Activities |
| 13.10.3 | Clinical Development |
| 13.10.3.1 | Clinical Trial Information |
| 13.10.4 | Safety and Efficacy |
| 13.11 | SAR-444656/KT-474: Sanofi/Kymera Therapeutics |
| 13.11.1 | Product Description |
| 13.11.2 | Other Developmental Activities |
| 13.11.3 | Clinical Development |
| 13.11.3.1 | Clinical Trial Information |
| 13.11.4 | Safety and Efficacy |
| 13.12 | Orismilast: UNION Therapeutics |
| 13.12.1 | Product Description |
| 13.12.2 | Other Developmental Activities |
| 13.12.3 | Clinical Development |
| 13.12.3.1 | Clinical Trials Information |
| 13.12.4 | Safety and Efficacy |
| 14 | Hidradenitis Suppurativa: Market Analysis |
| 14.1 | Key Findings |
| 14.2 | Market Outlook |
| 14.3 | Conjoint Analysis |
| 14.4 | Key Market Forecast Assumptions |
| 14.5 | Total Market Size of Hidradenitis Suppurativa in the 7MM |
| 14.6 | United States Market Size |
| 14.6.1 | Total Market Size of Hidradenitis Suppurativa in the United States |
| 14.6.2 | Market Size of Hidradenitis Suppurativa by Therapies in the United States |
| 14.7 | EU4 and the UK Market Size |
| 14.7.1 | Total Market Size of Hidradenitis Suppurativa in EU4 and the UK |
| 14.7.2 | Market Size of Hidradenitis Suppurativa by Therapies in EU4 and the UK |
| 14.8 | Japan Market Size |
| 14.8.1 | Total Market Size of Hidradenitis Suppurativa in Japan |
| 14.8.2 | Market Size of Hidradenitis Suppurativa by Therapies in Japan |
| 15 | Market Access and Reimbursement |
| 15.1 | United States |
| 15.1.1 | HUMIRA Reimbursement Program |
| 15.2 | Co-payment in the European Countries |
| 15.3 | The National Institute for Health and Care Excellence (NICE): UK |
| 15.4 | Institute for Quality and Efficiency in Healthcare (IQWiG): Germany |
| 15.5 | Haute Autorité de Santé (HAS): France |
| 15.6 | Italian Medicines Agency (AIFA): Italy |
| 15.7 | Spanish Agency of Medicines and Medical Products (AEMPS): Spain |
| 16 | Unmet Needs |
| 17 | SWOT Analysis |
| 18 | KOL Views |
| 19 | Appendix |
| 19.1 | Bibliography |
| 19.2 | Report Methodology |
| 20 | DelveInsight Capabilities |
| 21 | Disclaimer |
| 22 | About DelveInsight |
Related Reports
Hidradenitis Suppurativa Epidemiology Forecast
Hidradenitis Suppurativa Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted hidradenitis suppurativa epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hidradenitis Suppurativa Pipeline
Hidradenitis Suppurativa Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hidradenitis suppurativa companies, including InflaRx, Novartis, UCB, ChemoCentryx, Eli Lilly and Company, Janssen Biotech, AbbVie, Pfizer, Amgen, Incyte Corporation, CSL Behring, Kymera Therapeutics, Lytix Biopharma, Aclaris Therapeutics, Boehringer Ingelheim, Azora Therapeutics, among others.
Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atopic dermatitis companies including Arcutis Biotherapeutics, Amgen, Kyowa Kirin, Dermavant Sciences, Cara Therapeutics, Pfizer, Arena Pharmaceuticals, BioMimetix, Eli Lilly and Company, Aldeyra Therapeutics, Inc., Hangzhou Yirui Pharmaceutical Technology Co., Ltd, LEO Pharma, Corvus Pharmaceuticals, Inc., Brexogen Inc., Sanofi, Shaperon, UCB Pharma, Q32 Bio Inc., Akeso, Apogee Therapeutics, Inc., Allakos Inc., Biosion, Inc., among others.
Atopic Dermatitis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key atopic dermatitis companies, including Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, Dermira, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, and others. Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, Vanda Pharmaceuticals, Kyowa Kirin, Sanofi, KeyMed Biosciences, Asana BioSciences, Bristol-Myers Squibb, RAPT Therapeutics, Allakos, Novartis, BioMimetix, Shanghai Hengrui Pharmaceutical Co, Connect Biopharma, Pfizer, Evommune, Inc., Fresh Tracks Therapeutics, Biosion, Chia Tai Tianqing Pharmaceutical, Reistone Biopharma Company Limited, JW Pharmaceutical, Oneness Biotech, Alphyn Biologics, selectION, UNION Therapeutics, Ichnos Scien, among others.
Psoriasis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key psoriasis companies, including Mylan, Biocad, Bristol-Myers Squibb, Celltrion, Coherus BioSciences, Janssen Pharmaceuticals, Can-Fite Biopharma, Arcutis Biotherapeutics, Amgen, Iltoo Pharma, GlaxoSmithKline, Galectin Therapeutics, Evelo Biosciences, Galderma, BioMimetix JV, Menlo Therapeutics Inc., Aristea Therapeutics, UNION Therapeutics, MetrioPharm, Sienna Biopharmaceuticals, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
