New York, USA, Jan. 14, 2025 (GLOBE NEWSWIRE) -- Evolving Landscape of Cardiology Segment: Key Market Insights of Latest Published Rare Cardiovascular Diseases Report — Giant-Cell Arteritis, Pulmonary Arterial Hypertension, and Restrictive Cardiomyopathy | DelveInsight
Rare cardiovascular diseases are uncommon conditions affecting the heart and blood vessels, often with genetic or autoimmune origins. These diseases may have a prevalence of fewer than 1 in 2,000 individuals and are challenging to diagnose due to overlapping symptoms.
Rare cardiovascular diseases (RCDs) encompass a diverse group of uncommon heart and vascular conditions, such as Brugada syndrome, restrictive cardiomyopathy, and Takayasu arteritis. These diseases are often challenging to diagnose due to their rarity and overlapping symptoms with more common conditions. Treatments vary depending on the specific disease and may include medications, surgical interventions, implantable devices, or lifestyle modifications.
However, the lack of standardized treatment protocols and limited research compounds the difficulty in managing these conditions effectively. Patients with RCDs often face significant burdens, including delayed diagnosis, high medical costs, limited access to specialized care, and the emotional toll of living with an unpredictable condition. Comprehensive care involving multidisciplinary teams and advancements in genetic research and personalized medicine offer hope for improved outcomes and quality of life for those affected.
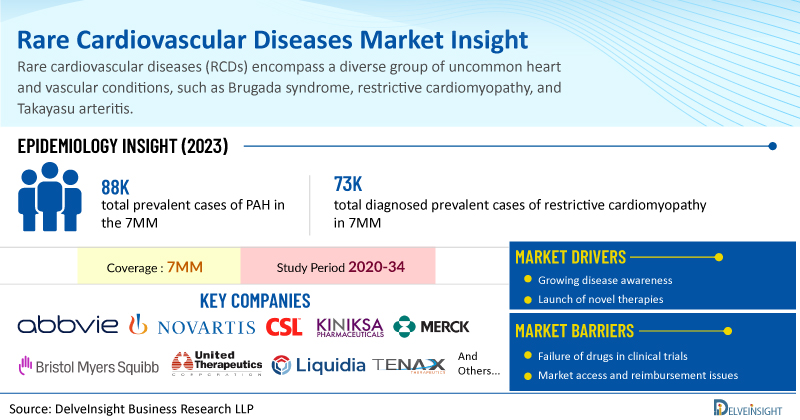
DelveInsight has expertise in the rare disease market with an experienced team handling the rare disease domain proficiently. DelveInsight has recently released a series of epidemiology-based market reports on rare cardiovascular diseases including Giant-Cell Arteritis, Pulmonary Arterial Hypertension, and Restrictive Cardiomyopathy. These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034 segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s deep dive into the assessment of these rare cardiovascular disease markets individually.
Giant-cell arteritis is classified as a type of large-vessel vasculitis but also involves medium and small arteries, particularly the superficial temporal artery, earning it the name temporal arteritis. It predominantly affects arteries like the ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries.
In 7MM, the United States accounted for the highest number of diagnosed prevalent cases of giant-cell arteritis, which is 54% of the diagnosed prevalent cases of giant-cell arteritis in 2023. According to DelveInsight’s analysis, across the 7MM, patients in the age segment >80 years accounted for the highest number of prevalent cases of giant-cell arteritis in 2023.
The main goal of treating GCA is to prevent permanent vision loss and manage blood vessel inflammation, which can cause tissue damage. If GCA is suspected in a patient, treatment should begin immediately, even before test results are confirmed. Corticosteroids are the standard treatment for temporal arteritis, and a doctor may start prescribing oral corticosteroids based on suspicion alone. Prednisone, the most commonly used corticosteroid, is proven effective in preventing vision loss. In most cases, prednisone produces a quick response, with blood inflammation markers typically improving within 2-4 weeks.
The dynamics of the giant-cell arteritis market are expected to change in the coming years. As per DelveInsight’s analysis, the market size for giant-cell arteritis reached USD 960 million in 2023 across the 7MM and it is expected to increase at a CAGR of 9.3% by 2034. This growth is mainly due to the growing geriatric population, the rise in the prevalence of cardiovascular disorders, technological advancements in the healthcare industry, and others.
Giant-Cell Arteritis Pipeline Therapies and Key Companies
- RINVOQ (upadacitinib): AbbVie
- COSENTYX (secukinumab): Novartis Pharmaceuticals
- Mavrilimumab: CSL/Kiniksa Pharmaceuticals
- TREMFYA (guselkumab): Johnson & Johnson/MorphoSys AG
Discover more about GCA drugs in development @ Giant-Cell Arteritis Clinical Trials
Pulmonary Arterial Hypertension Market
Pulmonary arterial hypertension is a rare and progressive condition marked by elevated blood pressure in the pulmonary arteries without an identifiable cause. The prevalence of pulmonary arterial hypertension has been rising, driven by the increasing incidence of cardiovascular diseases and arterial hypertension. Additionally, the expanding adult population, which is more susceptible to idiopathic pulmonary arterial hypertension, is contributing to the growing number of cases.
The total prevalent cases of PAH in the 7MM were ~88K in 2023. These cases are expected to increase during the forecast period i.e., 2024–2034. According to estimates based on DelveInsight’s epidemiology model for PAH, the subtype-specific distribution of the disease suggests that idiopathic/heritable make the majority of the PAH cases (42%), followed by connective tissue disease cases in the 7MM.
The US FDA has approved several therapies for PAH, including UPTRAVI (selexipag), REMODULIN (treprostinil), TYVASO (treprostinil), ORENITRAM (treprostinil), ADEMPAS (riociguat), among others. Additionally, some drugs approved for PAH are available in generic forms, such as REVATIO (sildenafil), ADCIRCA (tadalafil), LETAIRIS/VOLIBRIS (ambrisentan), VENTAVIS (iloprost), TRACLEER (bosentan), BERAPROST (TRK-100), and VELETRI (epoprostenol).
The primary treatments for PAH focus on dilating the pulmonary vasculature, which reduces pulmonary vascular resistance and, in turn, enhances right ventricular function, leading to improved functional capacity. The overarching goal of treatment is to enhance survival, quality of life, exercise capacity, alleviate symptom burden, and prevent clinical deterioration. Increasingly, risk stratification tools are being used to guide therapy and optimize these outcomes.
The dynamics of the PAH market are expected to change in the coming years. As per DelveInsight analysis, the market size of PAH was ~USD 5 billion in 2023 and it is expected to increase at a significant CAGR by 2034.
Despite significant progress in treatment options over the past few decades, pulmonary arterial hypertension remains a severe and rapidly progressing disease with high mortality. There is a need for curative therapies, as the current treatment regimen, which requires multiple injections per week and often leads to injection-site reactions, is not ideal for long-term health management. Several drugs, such as ralinepag, are being developed with high selectivity and potency, showing promising in vitro data with stronger antiproliferative and vasodilatory effects.
Additionally, agents targeting immune pathways, DNA repair, cellular senescence, and metabolic processes are in early development stages. As a result, the pipeline for pulmonary arterial hypertension is dynamic and promising, with the potential to significantly impact the current market during the forecast period (2024–2034).
Pulmonary Arterial Hypertension Pipeline Therapies and Key Companies
- Sotatercept (MK-7962): Merck/Bristol Myers Squibb
- Ralinepag: United Therapeutics
- YUTREPIA (inhaled treprostinil): Liquidia Technologies
- Imatinib (TNX-201): Tenax Therapeutics
- Vardenafil (RT234): Respira Therapeutics
- Seralutinib (GB002): Gossamer Bio
- L606 (liposomal treprostinil): Pharmosa Biopharm/Liquidia
- MK-5475: Merck Sharp & Dohme
- AV-101 (dry powder inhaled imatinib): Aerovate Therapeutics
- Treprostinil Palmitil (TPIP) (INS1009): Insmed
- LTP001: Novartis
- VASCULAN (ifetroban): Cumberland Pharmaceuticals
- Rodatristat Ethyl: Sumitomo Pharma (Enzyvant Therapeutics)
- CS1: Cereno Scientific
- LYNPARZA (olaparib): AstraZeneca
Dive deeper for rich insights into the Pulmonary Arterial Hypertension Clinical Trials
Restrictive Cardiomyopathy Market
Restrictive cardiomyopathy is a heart disorder where the heart muscle becomes stiff, limiting its ability to relax and fill with blood during the cardiac cycle's relaxation phase. This reduced flexibility hampers the heart's pumping efficiency, resulting in symptoms of heart failure.
DelveInsight’s analyst projects that the total diagnosed prevalent cases of restrictive cardiomyopathy in 7MM were approximately 73K in 2023 and these cases are further expected to increase during the forecasted period (2024–2034).
In the current treatment landscape for restrictive cardiomyopathy, efforts largely focus on symptom management and slowing disease progression rather than achieving meaningful disease modification. While various interventions are available, addressing the underlying molecular complexities remains a challenge, highlighting the need for innovative therapeutic approaches.
Pharmacological therapy is the cornerstone of RCM management, aimed at alleviating symptoms and enhancing cardiac function. Key medications such as diuretics, beta-blockers, calcium channel blockers, and ACE inhibitors are commonly used to relieve symptoms like dyspnea and fatigue by reducing fluid retention and optimizing heart performance.
The market for restrictive cardiomyopathy is projected to grow significantly. In 2023, the total market size in the 7MM was USD 26.09 million, with expectations of substantial growth by 2034, driven by a strong CAGR. In the 7MM, most of the market share was accommodated by RASI (ACEi/ARB) generating nearly USD 11.03 million in 2023. Among the 7MM, the US captured the highest market in 2023, covering a total of 69% market.
For a deeper understanding of the restrictive cardiomyopathy market landscape, explore the Restrictive Cardiomyopathy Market Outlook
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Rare Disease Consulting Services
Delveinsight’s comprehensive rare disease consulting services encompass rare disease consulting, epidemiology-based market assessment, and primary research projects aimed at obtaining elusive data through their esteemed KOL panel.
