SANTA MARIA, Calif, Oct. 12, 2011 (GLOBE NEWSWIRE) -- Hardy Diagnostics, an ISO certified biomedical firm, is pleased to announce the release of HardyCHROM™ Listeria, a chromogenic medium recommended for the selective isolation, differentiation, and enumeration of Listeria monocytogenes from food and environmental samples by colony color and appearance.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=10844
Listeria monocytogenes is a well-established agent of food poisoning. It can be found in uncooked meats and vegetables, as well as unpasteurized dairy products. Its ability to cause disease is due, in part, to the bacterium's ability to survive and grow at refrigerated temperatures. Clinical symptoms can range from flu-like illness to more serious conditions including meningitis, pneumonitis, septicemia and endocarditis. Listeria monocytogenes infections mainly occur in neonates, pregnant women, the elderly and immunocompromised individuals. Infections in pregnant women are a documented cause of spontaneous abortions and still births.
HardyCHROM™ Listeria is a chromogenic medium that allows for the rapid and reliable detection of Listeria monocytogenes. Current isolation methods for L. monocytogenes require multiple media types and can require up to ten days of incubation. With HardyCHROM™ Listeria, there is only a single broth enrichment step for a total incubation time of 48-72 hours.
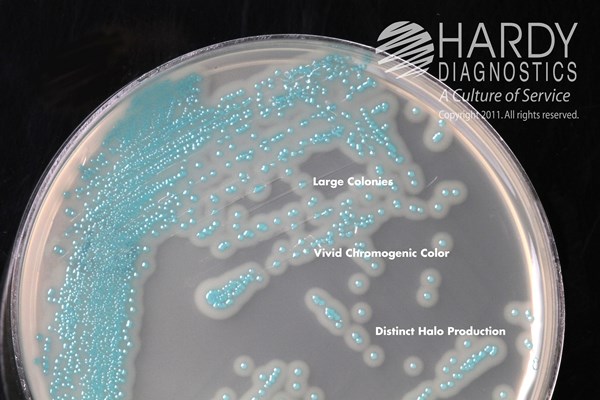
HardyCHROM™ Listeria contains specific chromogenic substrates that result in all Listeria species producing turquoise colored colonies when the substrate is hydrolyzed by specific bacterial enzymes. Further, this medium is able to detect the phospholipase activity specific to the two pathogenic Listeria species: L. monocytogenes and L. ivanovii. These two species will produce turquoise colored colonies surrounded by an opaque white halo within 48 hours. While L. ivanovii is rare in clinical samples, further tests are needed to definitively differentiate between these two species. Organisms other than Listeria are inhibited or grow as colorless or turquoise colonies without halos.
ABOUT HARDY DIAGNOSTICS
Hardy Diagnostics is an FDA licensed and ISO 13485 certified manufacturer of medical devices for microbiological procedures in both clinical and industrial laboratories. The company manufactures over 3,500 products for the culture and identification of bacteria and fungi from its Santa Maria, California headquarters. Currently over 7,000 laboratories are serviced by Hardy Diagnostics throughout the nation.
The company was founded in 1980 by Jay Hardy, a Clinical Laboratory Scientist from Santa Barbara, CA. Today, Hardy Diagnostics maintains eight distribution centers throughout the U.S. and exports products to over 40 foreign distributors.
The company's mission is to partner with its laboratory customers to prevent and diagnose disease. For more information, visit https://catalog.hardydiagnostics.com/cp_prod/CatNav.aspx?oid=10356&prodoid=G317