BRANCHBURG, N.J., May 03, 2022 (GLOBE NEWSWIRE) -- The U.S. Food and Drug Administration (FDA) has cleared ZEUS Scientific's dIFine® digital immunofluorescence system* for use with ZEUS' ANA HEp-2 indirect fluorescent antibody (IFA) assay.
The FDA-cleared ZEUS dIFine® digital immunofluorescence system is the next generation in IFA imaging and pattern recognition designed to acquire, analyze, display and store digital images of ZEUS IFA HEp-2 cells. An automated digital scanner with intelligent software, ZEUS dIFine quickly delivers positive/negative results and has been programmed to suggest eight common ANA HEp-2 patterns (homogenous, speckled, centromere, nucleolar, nuclear dots, nuclear membrane, cytoplasmic (ribosomal and mitochondrial)). dIFine also instantly locates and identifies mitotic cells to assist in pattern identification. The ability to quickly view and validate all negative samples with a single mouse click saves the user valuable time. The built-in pattern atlas can be used as a reference and is a valuable training tool allowing side-by-side comparison of images with ZEUS proprietary images aligned with ICAP nomenclature.
ANA HEp-2 IFA is the American College of Rheumatology (ACR) gold standard method for ANA testing. As a pioneer in IFA technology, ZEUS has continually delivered quality products and expertise in autoimmune serology that laboratories have relied upon since 1976.
For over 45 years, laboratories have trusted ZEUS Scientific to provide high-quality IFA ANA HEp-2 products. The ZEUS proprietary cell fixation process allows technologists to work with cells containing natural, unaltered antigens and provides clear, crisp images; excellent cell morphology; and a high number of mitotic cells, all of which makes pattern determination easier.
With this FDA clearance, laboratories can take their ANA IFA testing to the next level by pairing ZEUS' top-tier IFA ANA HEp-2 slides with the ZEUS dIFine automated immunofluorescence system. In addition, ZEUS is planning to submit to the FDA for review clinical data for automated negative/positive determination and detection of anti-dsDNA (nDNA/Crithidia) antibodies in 2022.
For more information, please visit the ZEUS dIFine website or contact the ZEUS sales team (sales@zeusscientific.com).
* FDA 510(k) cleared. All suggested results obtained with ZEUS dIFine must be confirmed by a trained operator.
About ZEUS Scientific
For over 45 years, ZEUS Scientific has been developing and manufacturing a wide variety of high-quality, in vitro diagnostic immunoassays for numerous infectious diseases and autoimmune disorders. Based in Branchburg, NJ, USA, ZEUS Scientific markets its products around the world with both a direct domestic sales team and an extensive international distribution network. ZEUS Scientific is a quality-minded, family-owned business that is fully certified and audited to: ISO 13485 (2016), MDSAP (Canada, Australia, USA), FDA QSR (1996: 21 CFR part 820), IVD 98/79/EEC and Health Canada MDR (SOR/98-282). Please visit www.zeusscientific.com to learn more about ZEUS Scientific and our products.
Related Images
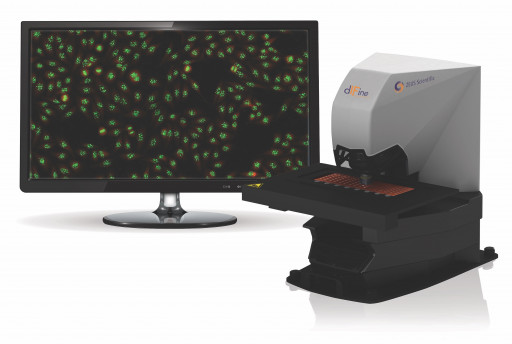
Image 1: ZEUS dIFIne
ZEUS dIFine IFA Imaging and Pattern Recognition System (HEp-2 ANA IFA)
This content was issued through the press release distribution service at Newswire.com.
Attachment
