EINDHOVEN, the Netherlands, Jan. 18, 2024 (GLOBE NEWSWIRE) -- ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative spinal cord stimulation therapies to restore movement, function, and independence in people with spinal cord injury (SCI), today announces the start of the HemON NL clinical feasibility study at Sint Maartenskliniek in Nijmegen, the Netherlands.
In late 2023, a study participant was implanted with an investigational ARC-IM Neurostimulator and Lead to assess the safety and effectiveness of ARC-IM Therapy to address hemodynamic instability after SCI. The surgery was performed by neurosurgeon Erkan Kurt, MD at Radboud University Medical Center, which has a neurosurgery department affiliated with Sint Maartenskliniek.
Building on the Swiss HemON clinical feasibility study, HemON NL prepares the Company for expected initiation of a global pivotal trial, called Empower BP, which is designed to provide the evidence necessary to submit a pre-market approval (PMA) to the US Food and Drug Administration (FDA) and other global regulatory authorities. In December 2022, the Company announced positive interim clinical results from its early feasibility studies showing improved regulation of blood pressure, thereby improving hemodynamic stability, after SCI.
The Company plans to enroll participants in both HemON and HemON NL as it finalizes the design of the Empower BP pivotal study.
“Sint Maartenskliniek has long been an outstanding research partner, and we are delighted to work with them on this new and exciting study to evaluate the use of ARC-IM Therapy to stabilize disruptive and potentially life-threatening fluctuations in blood pressure after SCI,” said Dave Marver, CEO of ONWARD. “This is an important but underappreciated recovery target after SCI. We hope this research and the expected upcoming pivotal study will shine a light on the importance of hemodynamic stability in this population.”
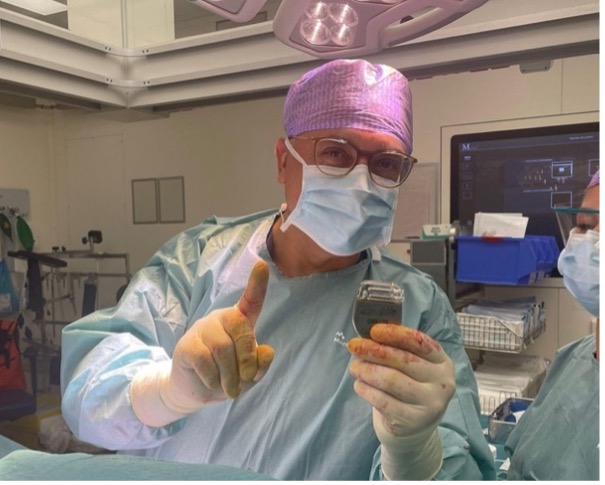
Dr. Erkan Kurt, MD implants his first ARC-IM Neurostimulator
The Principal Investigator of the HemON NL study is Dr. Ilse van Nes, a leading rehabilitation physician in the spinal cord injury department of Sint Maartenskliniek.
“The procedure went smoothly and the participant is responding well,” said Dr. van Nes. We are excited to monitor this and additional patients’ ongoing response to this groundbreaking therapy, which is designed to deliver programmed electrical stimulation to the area of the spine responsible for regulating blood pressure after SCI.”
ONWARD has received nine FDA Breakthrough Device Designations for its ARC-IM System, one of them for hemodynamic instability after SCI, inclusive of blood pressure regulation.
*All ONWARD devices and therapies, including but not limited to ARC-IM, ARC-EX, and ARC Therapy, alone or in combination with a BCI, are investigational and not available for commercial use.
About ONWARD Medical
ONWARD is a medical technology company creating therapies to restore movement, function, and independence in people with spinal cord injury (SCI) and movement disabilities. Building on more than a decade of science and preclinical research conducted at leading neuroscience laboratories, the Company has received nine Breakthrough Device Designations from the US Food and Drug Administration for its ARC Therapy™ platform.
ONWARD® ARC Therapy, which can be delivered by external ARC-EX or implantable ARC-IM systems, is designed to deliver targeted, programmed spinal cord stimulation. Positive results were presented in 2023 from the Company’s pivotal study, called Up-LIFT, evaluating the ability for transcutaneous ARC Therapy to improve upper extremity strength and function. The Company is now preparing regulatory approval submissions for ARC-EX for the US and Europe. In parallel, the Company is conducting studies with its implantable ARC-IM platform, which demonstrated positive interim clinical outcomes for improved blood pressure regulation, a component of hemodynamic instability, following SCI. Other ongoing studies include combination use of ARC-IM with a brain-computer interface (BCI) to address multiple symptoms of SCI.
Headquartered in Eindhoven, the Netherlands, ONWARD has a Science and Engineering Center in Lausanne, Switzerland and a US office in Boston, Massachusetts. The Company also has an academic partnership with .NeuroRestore, a collaboration between the Swiss Federal Institute of Technology (EPFL), and Lausanne University Hospital (CHUV).
For more information, visit ONWD.com, and connect with us on LinkedIn and YouTube.
For Media Enquiries:
Aditi Roy, VP Communications
For Investor Enquiries:
Khaled Bahi, Interim CFO
Disclaimer
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company’s or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties, and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release. All ONWARD devices and therapies referenced here, including but not limited to ARC-IM, ARC-EX, and ARC Therapy, are investigational and not available for commercial use.
