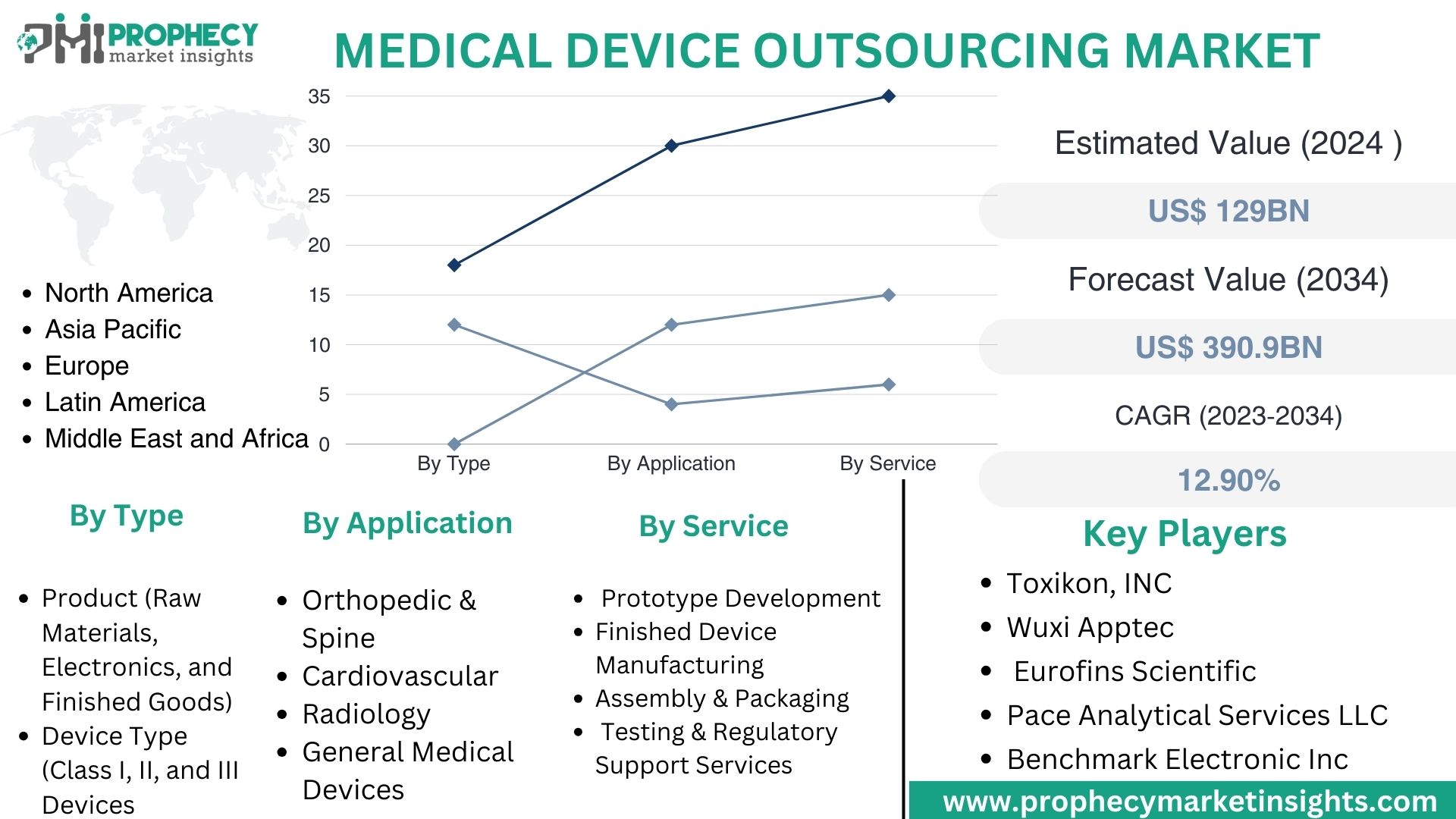
Covina, Feb. 23, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the Medical Device Outsourcing Market size was valued at about USD 129.0 Billion in 2024 and expected to grow at CAGR of 12.90% to extend a value of USD 390.9 Billion by 2034.”
What is Medical Device Outsourcing?
Market Overview:
Medical device outsourcing refers to the practice of delegating certain aspects of the production, development, testing, or distribution of medical devices to external third-party companies or organizations. In essence, it involves contracting out specific functions or processes related to medical device manufacturing and management to specialized service providers.
Medical device outsourcing include a wide range of tasks, such as the following:
- Design and Development: Companies may outsource the design and development of medical devices to specialized engineering firms or product design companies. This allows them to access expertise and resources that they may not have in-house.
- Manufacturing: Outsourcing manufacturing activities enables companies to leverage the capabilities of contract manufacturers (CMOs) or contract manufacturing organizations (CMOs) to produce medical devices. This can help reduce costs, increase production flexibility, and access specialized manufacturing technologies.
- Regulatory Compliance and Quality Assurance: Outsourcing regulatory compliance and quality assurance activities involves working with external consultants or firms to ensure that medical devices meet the necessary regulatory standards and quality requirements. This includes activities such as regulatory submissions, audits, and quality control processes.
- Supply Chain Management: Outsourcing supply chain management functions such as procurement, inventory management, and logistics allows companies to optimize their supply chain operations and reduce costs. This may involve working with third-party logistics (3PL) providers or suppliers to streamline the flow of materials and components.
- Clinical Trials and Testing: Companies often outsource clinical trials, testing, and validation studies to contract research organizations (CROs) or testing laboratories with the expertise and infrastructure to conduct rigorous scientific research and analysis. This helps ensure that medical devices are safe, effective, and compliant with regulatory requirements.
Medical device outsourcing offers several potential benefits for companies, including cost savings, access to specialized expertise, increased flexibility, and accelerated time-to-market. However, it also presents certain challenges and risks, such as maintaining quality control, protecting intellectual property, and managing relationships with external partners. As such, companies need to carefully evaluate their outsourcing strategies and establish effective partnerships to maximize the benefits of medical device outsourcing while mitigating potential risks.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/4882
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Top Leading Players in Medical Device Outsourcing Market:
- SGS SA
- Intertek Group PLC
- Wuxi Apptec
- TüvSüd AG
- Toxikon, INC.
- American Preclinical Services
- Eurofins Scientific
- Sterigenics International LLC
- Pace Analytical Services LLC.
- Charles River Laboratories International, Inc.
- North American Science Associates, Inc.
- IQVIA
- Accellent Inc.
- Celestica Inc.
- Benchmark Electronic Inc.
- Cadence Inc.
- Providien LLC
- Thermo Fischer Scientific Inc.
- West Pharmaceuticals Services Inc.
Market Dynamics:
Driving Factors:
- Outsourcing certain aspects of medical device production, such as manufacturing or assembly, can often be more cost-effective than handling them in-house. Outsourcing allows companies to leverage the expertise and economies of scale of specialized service providers, leading to cost savings.
- Access to Expertise: Medical device outsourcing enables companies to tap into the specialized knowledge, skills, and resources of external partners. These partners may have extensive experience in specific areas such as design, regulatory compliance, or clinical trials, which can help companies accelerate product development and navigate complex regulatory requirements.
- Focus on Core Competencies: Outsourcing non-core functions allows medical device companies to focus their resources and attention on core competencies such as research and development, innovation, and strategic planning. By delegating routine tasks to external partners, companies can improve efficiency and allocate resources more effectively.
- Outsourcing provides medical device companies with greater flexibility and scalability in response to changing market demands, production volumes, or resource requirements. Companies can adjust their outsourcing arrangements based on fluctuations in demand, without the need for significant investments in infrastructure or personnel.
Restrain Factors:
- Quality Control
- Intellectual Property Protection
- Dependency on External Partners
Emerging Trends and Opportunities in Medical Device Outsourcing Market:
- As medical devices become more complex and sophisticated, there is a growing demand for specialized services such as product design, prototyping, regulatory consulting, and clinical trial management. Outsourcing companies that offer expertise in niche areas are well-positioned to capitalize on this demand.
- Contract development and manufacturing organizations (CDMOs) are playing an increasingly important role in the medical device outsourcing market. These companies offer end-to-end services, from product development and manufacturing to packaging and distribution, providing comprehensive solutions to medical device companies.
- Technological advancements, such as additive manufacturing (3D printing), artificial intelligence (AI), and digital health solutions, are driving innovation in the medical device industry. Outsourcing companies that leverage these technologies to enhance product development, manufacturing processes, and patient outcomes are poised for success.
- Medical device companies are increasingly outsourcing non-core functions such as supply chain management, logistics, and post-market surveillance to focus on their core competencies. Outsourcing these functions allows companies to streamline operations, reduce costs, and improve efficiency.
- The medical device outsourcing market is witnessing globalization, with outsourcing services being offered across geographical boundaries. This trend enables medical device companies to access a diverse pool of talent, resources, and manufacturing facilities, driving innovation and competitiveness.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/4882
Challenges of Medical Device Outsourcing Market:
- Long-term outsourcing arrangements or over-reliance on a single vendor can lead to vendor lock-in, limiting flexibility, innovation, and competitiveness.
- Diversifying outsourcing relationships, fostering competition among vendors, and periodically reassessing outsourcing strategies can mitigate the risk of vendor lock-in.
- Outsourcing involves sharing sensitive data, including patient information and proprietary business data, with external partners.
- Protecting data security and ensuring compliance with privacy regulations such as HIPAA (Health Insurance Portability and Accountability Act) or GDPR (General Data Protection Regulation) is critical to maintaining trust, confidentiality, and regulatory compliance.
Detailed Segmentation:
Medical Device Outsourcing Market, By Type:
-
-
- Product (Raw Materials, Electronics, and Finished Goods)
- Device Type (Class I, II, and III Devices)
-
Medical Device Outsourcing Market, By Application:
-
-
- Orthopedic & Spine
- Cardiovascular
- Radiology
- General Medical Devices
-
Medical Device Outsourcing Market, By Service:
-
-
- Prototype Development
- Finished Device Manufacturing
- Assembly & Packaging
- Testing & Regulatory Support Services
-
Medical Device Outsourcing Market, By Region:
-
-
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
-
- Asia Pacific
-
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Mexico
- Rest of Latin America
-
- Middle East & Africa
-
- GCC
- Israel
- South Africa
- Rest of Middle East & Africa
-
- North America
-
Regional Analysis:
Regional insights highlight the diverse market dynamics, regulatory landscapes, and growth drivers shaping the Medical Device Outsourcing Market across different geographic areas. Understanding regional nuances and market trends is essential for stakeholders to capitalize on emerging opportunities and drive market expansion in the Medical Device Outsourcing sector.
Asia Pacific market is estimated to witness the fastest share over the forecast period as this region is emerging as a major hub for medical device outsourcing due to factors such as cost advantages, skilled labor pool, and improving infrastructure. The region offers attractive opportunities for medical device manufacturers to outsource various functions including manufacturing, research and development, design, regulatory compliance, and clinical trials.
Report scope:
| Attribute | Details |
| Market Size 2024 | US$ 129.0 Billion |
| Projected Market Size 2034 | US$ 12.90 Billion |
| CAGR Growth Rate | 12.90% |
| Base year for estimation | 2023 |
| Forecast period | 2024 – 2034 |
| Market representation | Revenue in USD Million & CAGR from 2024 to 2034 |
| Market Segmentation | By Type - Product (Raw Materials, Electronics, and Finished Goods), Device Type (Class I, II, and III Devices) By Application – Orthopedic & Spine, Cardiovascular, Radiology, and General Medical Devices By Service – Prototype Development, Finished Device Manufacturing, Assembly & Packaging, and Testing & Regulatory Support Services |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key highlights of the Medical Device Outsourcing Market:
- The medical device outsourcing market has been experiencing steady growth due to factors such as increasing healthcare expenditure, technological advancements, and rising demand for cost-effective manufacturing solutions.
- Medical device outsourcing offers cost-efficient solutions for manufacturers by reducing overhead costs, labor expenses, and infrastructure investments associated with in-house production.
- Outsourcing firms often possess specialized expertise in areas such as design, engineering, regulatory compliance, and manufacturing processes, allowing medical device companies to access skills and resources that may not be available internally.
- Outsourcing partners help medical device companies navigate complex regulatory landscapes and ensure compliance with stringent quality and safety standards imposed by regulatory authorities such as the FDA (Food and Drug Administration) in the United States marking regulations in Europe.
- Outsourcing enables medical device manufacturers to expand their market reach by accessing outsourcing partners with established networks, distribution channels, and market presence in various regions across the globe.
- The medical device outsourcing market is characterized by strategic partnerships and collaborations between manufacturers and outsourcing firms, allowing for shared expertise, risk mitigation, and innovation throughout the product development lifecycle.
- Rapid advancements in technology, such as additive manufacturing, robotics, artificial intelligence, and digital health solutions, are driving innovation and efficiency in the medical device outsourcing market.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/4882
Explore More Insights:
- Veterinary Imaging Market - Trends, Analysis and Forecast till 2034
- Colorectal Cancer Therapeutics Market – Trends, Analysis and Forecast till 2034
- Blood Glucose Monitoring Devices Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
