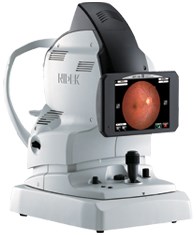
Photo Release -- NIDEK and MARCO Announce FDA 510(K) Clearance for the AFC-330 - Automated Fundus Camera
May 15, 2012 14:44 ET
|
NIDEK, Inc.
FREMONT, Calif. and JACKSONVILLE, Fla., May 15, 2012 (GLOBE NEWSWIRE) -- NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, announces the FDA 510(k)...

NIDEK Announces Enhanced Alliance With MARCO Ophthalmic by Expanding the Sales Distribution Channels of New Fundus Camera
February 27, 2012 08:00 ET
|
NIDEK, Inc.
FREMONT, Calif. and JACKSONVILLE, Fla., Feb. 27, 2012 (GLOBE NEWSWIRE) -- NIDEK announces a market expansion of their fundus camera distribution by granting MARCO Ophthalmic exclusive rights to sell...

NIDEK Announces FDA Clearance for Multicolor Pattern Scan Laser at American Society of Retina Specialists Meeting in Boston
August 23, 2011 09:00 ET
|
NIDEK, Inc.
BOSTON, Aug. 23, 2011 (GLOBE NEWSWIRE) -- NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, announces their FDA Clearance for the MC-500 Vixi, Multicolor...

Eyefinity/OfficeMate and NIDEK Announce Equipment Interface for ExamWRITER Version 9.5
December 02, 2010 16:08 ET
|
NIDEK, Inc.
RANCHO CORDOVA, Calif., Dec. 2, 2010 (GLOBE NEWSWIRE) -- Eyefinity®/OfficeMate®, the leader in electronic medical records, and NIDEK, a global leader in the design, manufacturing and...

NIDEK and Compulink Announce Full Integration With NIDEK's AFC Pro Photographer
October 05, 2010 12:51 ET
|
NIDEK, Inc.
FREMONT and WESTLAKE VILLAGE, Calif., Oct. 5, 2010 (GLOBE NEWSWIRE) -- NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, and Compulink, a leader in fully...

NIDEK's Me 1200 Lens Edger Wins 2010 "SILMO d'Or" Award
October 04, 2010 15:45 ET
|
NIDEK, Inc.
FREMONT, Calif. and HAUPPAUGE, N.Y., Oct. 4, 2010 (GLOBE NEWSWIRE) -- NIDEK is proud to announce that its new Multifunction Lens Edger Me 1200 was awarded the prestigious "SILMO d'Or" prize in the...

NIDEK and MaximEyes by First Insight Announce Direct Integration With AFC Pro Photographer Automated Fundus Camera
September 20, 2010 16:02 ET
|
NIDEK, Inc.
FREMONT, Calif. and HILLSBORO, Ore., Sept. 20, 2010 (GLOBE NEWSWIRE) -- NIDEK, a leader in designing and manufacturing equipment for the diagnosis and treatment of retinal diseases, glaucoma, and...

NIDEK Gains FDA Clearance for ORION, a New Auto-Retinal Imaging Device
April 28, 2007 17:49 ET
|
NIDEK, Inc.
FREMONT, Calif., April 28, 2007 (PRIME NEWSWIRE) -- NIDEK, Inc., a global leader in laser and diagnostic instrumentation for the optical and eye care industry, announced today that the U. S. Food...
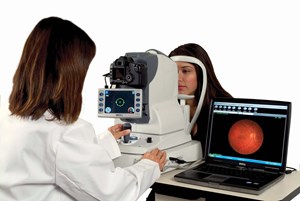
Photo Release -- NIDEK Announces World's First Auto-Focus, Auto-Alignment Fundus Camera
April 03, 2007 08:00 ET
|
NIDEK, Inc.
FREMONT, Calif., April 3, 2007 (PRIME NEWSWIRE) -- NIDEK Inc. announces the release of the world's first auto-focus, auto-alignment non-mydriatic fundus camera. The AFC-210 Pro Photographer takes true...

NIDEK Gains FDA Approval for Hyperopia and Hyperopic Astigmatism Module on EC-5000 Excimer Laser
October 17, 2006 19:16 ET
|
NIDEK, Inc.
FREMONT, Calif., Oct. 17, 2006 (PRIMEZONE) -- NIDEK, Inc. a global leader in laser and diagnostic instrumentation for the optical and eye care industry, announced today that the U.S. Food & Drug...