Dublin, June 06, 2024 (GLOBE NEWSWIRE) -- The "Global Biologics Safety Testing Market by Product & Service (Consumables, Instrument, Services), Test Type (Mycoplasma, Sterility, Endotoxin, Bioburden, Virus Safety), Application (Vaccines, mAbs, Cell & Gene Therapy, Blood Products) - Forecast to 2029" report has been added to ResearchAndMarkets.com's offering.
The biologics safety testing market is witnessing substantial growth with projections indicating a rise from USD 4.2 billion in 2024 to USD 7.2 billion by 2029, growing at a CAGR of 11.1%
This growth is predominantly fueled by the intensified development and production of novel biologics and an enhanced demand for cell and gene therapies attributed to the increasing prevalence of chronic diseases. Industry leaders anticipate that these factors will continue to underpin the expansion of the biologics safety testing market over the forecast period.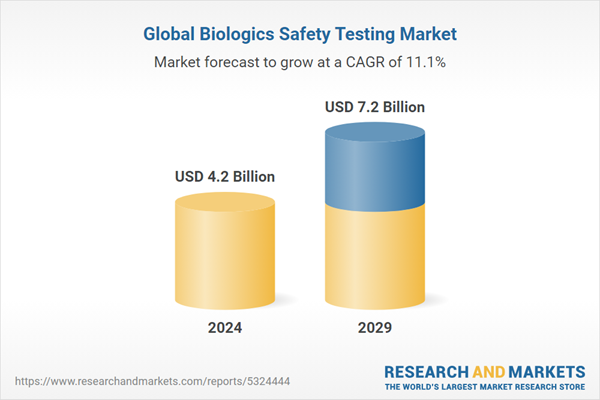
In 2023, the development and manufacturing of monoclonal antibodies constituted the largest application segment within the biologics safety testing market. The burgeoning investments in novel biological therapeutics, coupled with the rising necessity for biological treatments for chronic conditions, are propelling the significance and growth of this segment.
United States Maintains Market Dominance
With a comprehensive biopharmaceutical market and leadership in research investments, the United States continues to assert its dominance in the biologics safety testing market. Renowned for its robust healthcare infrastructure, the US market benefits from an abundance of biopharmaceutical entities and a surge in biotechnological research activities, catalyzing market expansion. Approvals for biosimilars also contribute substantially to the country's market proliferation.
Comprehensive Research Insights
Evaluating a range of pivotal market aspects such as drivers, restraints, challenges, and opportunities that influence market dynamics, the report thoroughly analyzes the competitive landscape and the broader environment of the biologics safety testing domain. Key industry players are scrupulously examined to offer insights into their business operations, product portfolios, strategic initiatives, and significant industry movements, including product launches and strategic partnerships.
Benefits for Stakeholders
The substantial benefits of the report are its value to stakeholders, helping them to understand the competitive landscape and informing their strategic decisions. It facilitates market leaders and new entrants by providing detailed revenue approximations, insights into market drivers, and an understanding of key market challenges and opportunities. The report is instrumental in guiding stakeholders to comprehend market conditions and equipping them with crucial information on market developments.
The biologics safety testing market is set for continued growth, backed by innovation and increased demand in the biopharmacological sector. Stakeholders can anticipate a deeper understanding of the market landscape and leverage opportunities generated in this dynamic field. The expansive and detailed analysis within this market report will undoubtedly serve as a critical tool for those embedded in the biologics safety testing ecosystem.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 387 |
| Forecast Period | 2024 - 2029 |
| Estimated Market Value (USD) in 2024 | $4.2 Billion |
| Forecasted Market Value (USD) by 2029 | $7.2 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
Companies Featured
- Thermo Fisher Scientific Inc.
- Charles River Laboratories
- Laboratory Corporation of America Holdings
- F. Hoffmann-La Roche Ltd.
- Merck KGaA
- Sartorius AG
- Lonza
- Fujifilm Corporation
- Biomérieux
- Maravai Lifesciences
- Wuxi Apptec
- Sgs Société Générale De Surveillance SA.
- Sotera Health
- Samsung Biologics
- Genscript
- Agilent Technologies, Inc.
- Syngene International Limited
- Eurofins Scientific
- Bio-Rad Laboratories, Inc.
- Qiagen
- Promega Corporation
- Catalent, Inc.
- Associates of Cape Cod, Inc.
- Clean Biologics
- Pathoquest
- Pacific Biolabs
- Arl Bio Pharma, Inc.
- Frontage Labs
- Creative Biogene
- Advaxia Biologics
For more information about this report visit https://www.researchandmarkets.com/r/1bstn4
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
