New York, June 10, 2024 (GLOBE NEWSWIRE) -- Market Overview
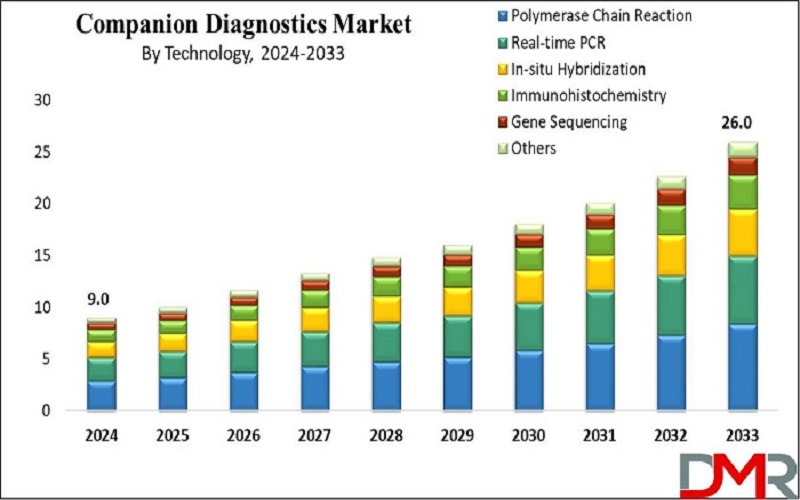
The Global Companion Diagnostics Market size is expected to reach USD 9.0 billion by 2024 and is further anticipated to reach USD 26.0 billion by 2033 at a CAGR of 12.5 % according to Dimension Market Research.
Companion diagnostic is a specialized test designed to ensure the safe & effective use of a specific biological product or drug. These tests are commonly used in cancer diagnosis to identify biomarkers, which help recommend the most suitable drug for personalized treatment tailored to each patient's specific condition.
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/companion-diagnostics-market/request-sample/
The global companion diagnostics market focuses on diagnostic tests utilized by patients with various medical conditions. These tests assist healthcare professionals in making informed decisions about appropriate treatment options for individual patients.
Important Insights
- Market Progression Overview: The global companion diagnostics market is projected to expand by USD 26.0 billion, with a CAGR of 12.5% from 2025 to 2033.
- Definition: Companion diagnostics are tests used alongside specific medical treatments to help doctors determine if a particular therapy is suitable for a patient.
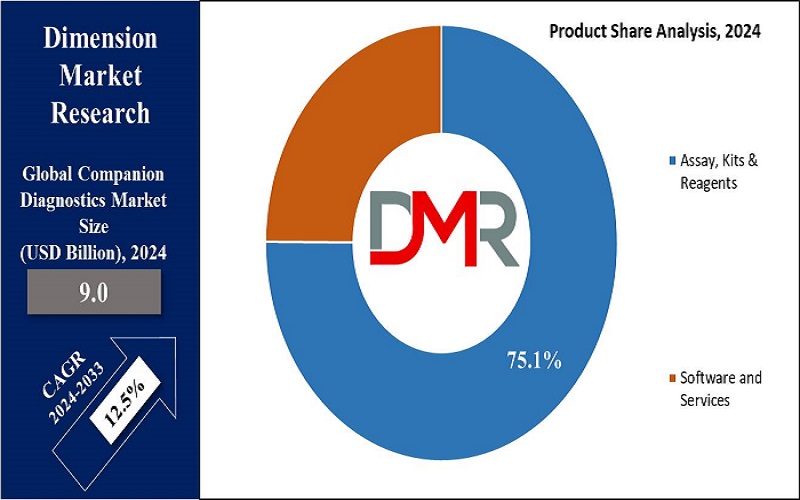
- Product Insights: Assay, Kits, and reagents are expected to lead the global companion diagnostics market, commanding the largest revenue share of 75.1% by 2024.
- Technology Insights: Polymerase chain reaction (PCR) technology is anticipated to dominate the market, holding the highest revenue share of 38.9% in 2024.
- Indication Insights: Cancer is projected to be the leading indication in the global market, capturing the largest revenue share in 2024.
- Sample Type Insights: Tissue samples are forecasted to dominate the market based on sample type, with the largest market share in 2024.
- End-User Insights: Pharmaceutical and biopharmaceutical companies are expected to be the primary end-users in the global market, holding the largest market share in 2024.
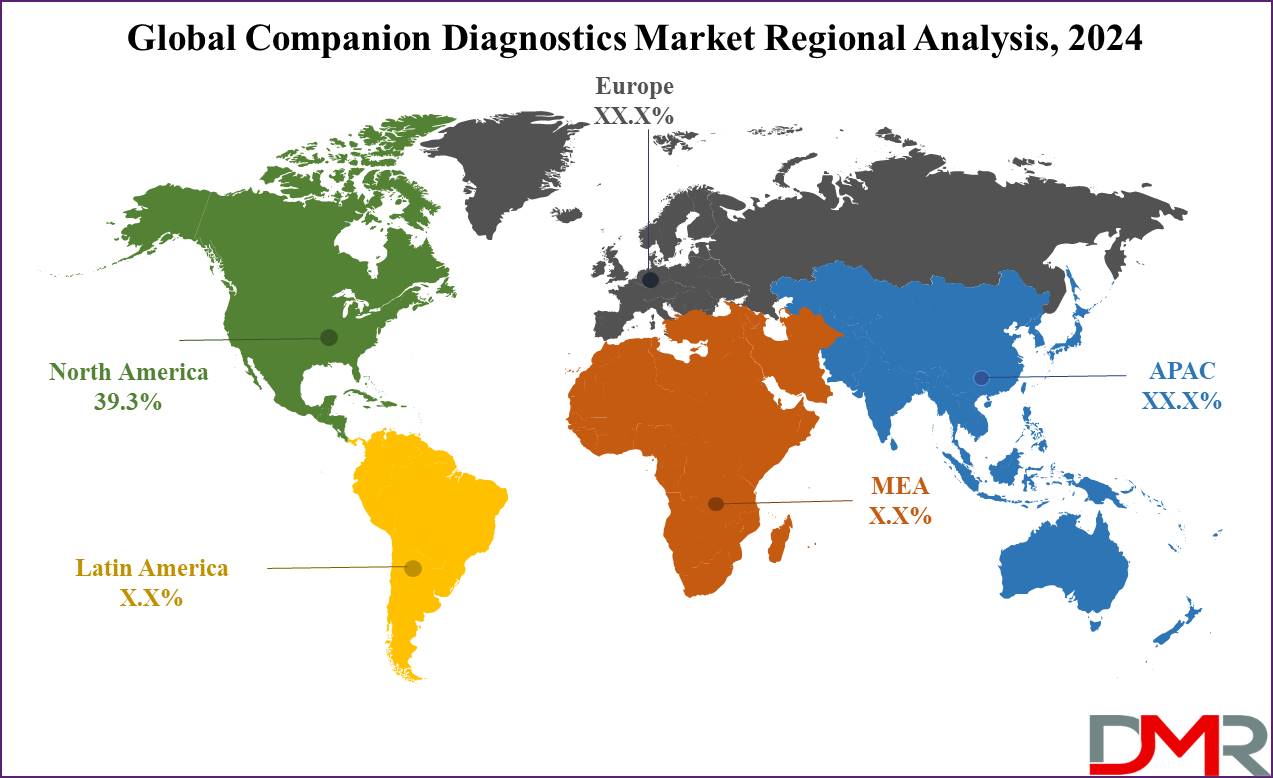
- Regional Insights: North America is projected to lead the global companion diagnostics market, accounting for 39.3% of the market share in 2024.
Latest Trends
- Pharmaceutical companies & diagnostic firms are increasingly collaborating to co-develop companion diagnostics with new drugs.
- Governments and non-profit organizations are partnering with private companies to support companion diagnostics research and development.
- Companies are expanding their companion diagnostic offerings to emerging markets, driven by growing healthcare infrastructure and increasing demand for personalized medicine.
Competitive Landscape
- Major pharmaceutical companies, such as Abbott Laboratories Inc. and Agilent Technologies Inc., are actively engaged in the companion diagnostic market, seeking to develop these diagnostics to gain a competitive edge.
- These pharmaceutical firms often collaborate with diagnostic companies to co-develop and commercialize companion diagnostics.
- Diagnostic companies, including Roche Diagnostics, Qiagen, and Agilent Technologies, focus on the development and commercialization of diagnostic tests, including companion diagnostics, leveraging expertise in various diagnostic technologies like genomics and in vitro diagnostics.
Some of the prominent market players:
- Abbott Laboratories Inc.
- Agilent Technologies Inc.
- Hoffmann-La Roche Ltd
- Biomerieux SA
- Qiagen NV
- Siemens Healthcare
- Thermo Fisher Scientific Inc.
- Danaher Corporation
- Almac Group
- Illumina Inc.
- Myriad Genetics Inc.
- Guardant Health Inc.
- Biogenex Laboratories Inc
- Others
Transform your business approach with strategic insights from our report. Get in touch to request our brochure today! : https://dimensionmarketresearch.com/report/companion-diagnostics-market/download-reports-excerpt/
Companion Diagnostics Market Scope
| Report Highlights | Details |
| Market Size (2024) | USD 9.0 Bn |
| Forecast Value (2033) | USD 26.0 Bn |
| CAGR (2024-2033) | 12.5% |
| Leading Region in terms of Revenue Share | North America |
| Percentage of Revenue Share by Leading Region | 39.3% |
| Historical Data | 2017 - 2022 |
| Forecast Data | 2025 – 2033 |
| Base Year | 2023 |
| Estimate Year | 2024 |
| Segments Covered | By Product, By Technology, By Indication, By Sample Type, By End User |
| Regional Coverage | North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
Market Analysis
Assays, Kits & Reagents segment is projected to dominate the global companion diagnostics market, holding 75.1% of the market share by the end of 2024 based on product, and is expected to exhibit the fastest CAGR from 2024 to 2033. The growth of this segment is due to their detailed solutions for conducting diagnostic tests.
These products are typically available in packaged kits that include all necessary components such as primers, probes, enzymes, & buffers. This simplifies the process for researchers & healthcare professionals by eliminating the need to source each component separately.
Companion Diagnostics Market Segmentation
By Product
- Assay, Kits & Reagents
- Software & Services
By Technology
- Polymerase Chain Reaction (PCR)
- Real-time PCR (RT-PCR)
- In-situ Hybridization (ISH)
- Immunohistochemistry (IHC)
- Gene Sequencing
- Others
By Indication
- Cancer
- Neurological disease
- Infectious Disease
- Other
By Sample Type
- Tissue Samples
- Blood Samples
- Others
By End User
- Pharmaceutical & Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
- Others
Purchase the Competition Analysis Dashboard Today: https://dimensionmarketresearch.com/checkout/companion-diagnostics-market/
Drivers
- Ongoing technological advancements, especially in genomics, sequencing technologies, and diagnostic tools, are driving the development of more precise and efficient diagnostic tests, fueling the growth of the companion diagnostic market.
- Integrating artificial intelligence and machine learning into diagnostic processes is revolutionizing the field by providing deeper insights and faster results, further supporting market expansion.
- The shift towards personalized medicine, increasingly recognized in the healthcare industry, is a significant driver for the companion diagnostic market.
- Physicians & patients seek treatment plans tailored to individual genetic profiles, and companion diagnostics meet this need, promoting market growth.
Restraints
- High investment is required for biomarker discovery, development, and validation due to the high attrition rate of drugs in clinical trials, with around 30% failing in phase III, posing substantial financial challenges for diagnostic manufacturers.
- Conducting diagnostic trials and adhering to stringent regulatory standards require significant investment, making it difficult for smaller companies to innovate and develop new biomarkers, thereby restraining market growth.
- The companion diagnostic process is technically complex and requires trained, skilled professionals for accurate execution. A lack of awareness among healthcare professionals about the latest advancements in companion diagnostic tests leads to operational knowledge gaps in clinical laboratories, obstructing the adoption of these diagnostics and restraining market growth.
Opportunities
- The use of companion diagnostics is increasing as leading pharmaceutical companies employ these tests to select suitable patient populations for drug trials and assess treatment efficacy.
- This strategy facilitates the introduction of new drugs and treatment plans to the healthcare market, creating more growth opportunities.
- Regulatory bodies like the FDA are creating more defined and streamlined approval processes for companion diagnostics. These clearer regulatory processes encourage increased investment from companies eager to develop companion diagnostics.
- Enhanced clarity in the regulatory environment reduces uncertainty and time for bringing new companion diagnostics to market, accelerating innovation and availability.
Regional Analysis
North America is expected to lead the global companion diagnostic market, holding a market share of 39.3% by 2024. This dominance can be attributed to the region's concentration of major pharmaceutical and biotechnology companies. These companies actively engage in pharmaceutical research & often collaborate with research institutions. Their strong presence contributes significantly to North America's leadership in the global companion diagnostics market.
Additionally, the region benefits from a well-established regulatory framework, particularly in the United States, where bodies like the Food & Drug Administration (FDA) provide clear guidelines for drug production.
By Region
North America
- The U.S.
- Canada
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/companion-diagnostics-market/request-sample/
Recent Developments
- February 2024: Hoffmann-La Roche Ltd collaborated with PathAI to develop AI-driven pathology algorithms to enhance their companion diagnostics.
- November 2023: Amoy Diagnostics Co., Ltd. partnered with Cell Signaling Technology to advance precision oncology through the development of companion diagnostics in China.
- August 2023: Agilent Technologies, Inc. received European IVDR Certification for their Companion Diagnostic Assay.
- August 2023: QIAGEN obtained FDA approval for a companion diagnostic to aid in therapy selection for gastrointestinal stromal tumors in collaboration with Blueprint Medicines’ AYVAKIT.
- March 2023: Hoffmann-La Roche Ltd received FDA approval to expand the label of the VENTANA PD-L1 (SP263) Assay, assisting in identifying lung cancer patients eligible for Libtayo.
- September 2022: Thermo Fisher Scientific Inc. announced FDA approval for the Oncomine Dx Target Test, the first NGS-based companion diagnostic to aid therapy selection for patients with RET mutations/fusions in thyroid cancers.
Browse More Related Reports
- Infusion Pump Market is expected to reach a value of USD 6.1 billion in 2023, and it is further anticipated to reach a market value of USD 13.3 billion by 2032 at a CAGR of 9.0%.
- Gabapentin Market will grow to USD 2.9 billion in 2023, and it is further predicted to reach a market value of USD 5.1 billion by 2032 at a CAGR of 6.6%.
- End-Stage Renal Disease Market is expected to reach a value of USD 119.4 billion in 2023, and it is further anticipated to reach a market value of USD 398.7 billion by 2032 at a CAGR of 14.2%.
- In-Vitro Toxicology Market is expected to reach a value of USD 36.4 billion in 2023, and it is further anticipated to reach a market value of USD 104.8 billion by 2032 at a CAGR of 12.5%.
- Infectious Disease In-vitro Diagnostics Market is expected to reach a value of USD 93.2 billion in 2023, and it is further anticipated to reach a market value of USD 140.6 billion by 2032 at a CAGR of 4.7%.
- Healthcare Analytics Testing Service Market will grow to USD 13.9 billion in 2023 and is predicted to show subsequent growth with a market value of USD 30.2 billion by the end of 2032 at a CAGR of 9.0%.
- Home Healthcare Market is expected to reach a value of USD 414.4 billion in 2023, and it is further anticipated to reach a value of USD 879.5 billion by 2032 at a CAGR of 19.3%.
- Nuclear Medicine Market is expected to reach a value of USD 10.8 billion in 2023, and it is further anticipated to reach a market value of USD 30.6 billion by 2032 at a CAGR of 12.2%.
- Microbial Fermentation Technology Market is expected to reach a value of USD 36.5 billion in 2023, and it is further anticipated to reach a market value of USD 62.3 billion by 2032 at a CAGR of 6.1%.
- Cancer Immunotherapy Market is expected to reach a value of USD 132.6 billion in 2023, and it is further anticipated to reach a market value of USD 298.7 billion by 2032 at a CAGR of 9.4%.
About Dimension Market Research (DMR):
Dimension Market Research (DMR) is a market research and consulting firm based in India & US, with its headquarters located in the USA (New York). The company believes in providing the best and most valuable data to its customers using the best resources analysts into work, to create unmatchable insights into the industries, and markets while offering in-depth results of over 30 industries, and all major regions across the world.
We also believe that our clients don’t always want what they see, so we provide customized reports as well, as per their specific requirements to create the best possible outcomes for them and enhance their business through our data and insights in every possible way.
