Pune, July 15, 2024 (GLOBE NEWSWIRE) -- Pharmacovigilance Market Size & Growth Analysis:
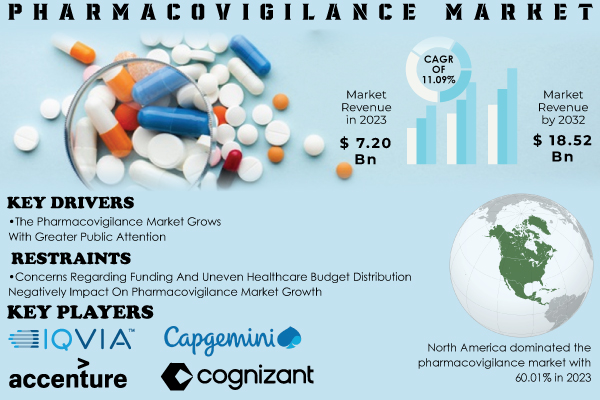
“According to SNS Insider Research, The Pharmacovigilance Market size was valued at US$ 7.20 billion in 2023, and estimated to reach US$ 18.52 billion by 2032 with CAGR of 11.09% over the forecast period 2024-2032.”
The increase in demand for more safety monitoring is driven by growing polypharmacy, especially in chronic diseases such as Parkinson’s – a neurodegenerative disorder that primarily impacts people 60 years of age and older, second most prevalent in America. However, 5-10% of patients are early-onset cases. Although it is estimated that as many as 500,000 people are diagnosed, the true figure may be much greater due to poor diagnosis and misdiagnosis and the increasing incidence of adverse drug reactions. In addition, there is a large drug development pipeline and increasing events in personalized medicines and biosimilars field. For example, according to the National Library of Medicine article of 2023, the use of five or more medications, known as polypharmacy, has been on the rise in adults across the U.S. In adults, this growth has increased from 8.2% to 17.1%, an average annual growth rate of about 2.9%. This growth is particularly significant in the elderly, those with heart disease or diabetes, and is particularly noticeable between men and Mexican Americans and non-Hispanic Blacks.
Effective pharmacovigilance requires a strong underlying data infrastructure which is essential for the supply chain in different sort (i.e., data) to flow efficiently from many sources. Nonetheless, problems like delays and issue illustrations could be a barrier.
Outsourcing in pharmacovigilance is well catching up the trend as companies looks to save cost on non-core operations. At the same time, it is experiencing huge technological progressions with AI and big data disrupting traditional processes.
Get a Sample Report of Pharmacovigilance Market@ https://www.snsinsider.com/sample-request/3095
Major Players Analysis Listed in this Report are:
- ArisGlobal,IQVIA
- Accenture
- Cognizant
- IBM
- Clinquest Group B.V. (Linical Americas)
- Laboratory Corporation of America Holdings
- Capgemini
- ICON plc.
- ITClinical
- ClinChoice (formerly FMD K&L)
- TAKE Solutions Limited
- Wipro
- Parexel International (MA) Corporation
- BioClinica Inc. (Clario)
- United BioSource LLC
- Other Players
There are challenges though funding limitations and skill shortages while the pharmacovigilance market has some great drivers for growth with the investments in training and infrastructure are necessary to have an impact on overcoming these hurdles.
The competitive environment is dynamic, and M&A activity remains an ongoing strategy. This, however, is counterbalanced by strict regulations governing TPO products; mainly in North American and European provinces influencing the pharmacovigilance market structure.
Overall, the pharmacovigilance market is bound to soar high with drug complexity and ADRs going forward, technological advancements around the corner as well as burgeoning regulatory focus on medication safety.
Pharmacovigilance Market Report Scope:
| Report Attributes | Details |
| Market Size in 2023 | US$ 7.20 Bn |
| Market Size by 2032 | US$ 18.52 Bn |
| CAGR | CAGR of 11.09% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Key Regional Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]). Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
Do you have any specific queries or need any customization research on Pharmacovigilance Market, Enquire Now@ https://www.snsinsider.com/enquiry/3095
Key Market Drivers:
- Rising ADRs and multidrug regimens
- Expanding product pipeline
- Innovations in technology (AI, big data)
- Outsourcing trend
Key Market Challenges:
- Problems of Data quality and availability
- Talent Shortage
- Strict regulatory space
Key Market Opportunities:
- AI & Machine learning in the process
- Further development of pharmacovigilance services
- Growth in emerging markets
The Pharmacovigilance Market - A Landscape of Services & Stages
By Product Life Cycle, the most important phase is post-marketing surveillance (phase IV), which represents a large part of the market. It aims to discover unknow side effects of marketed drugs in large number of treated patients.
By Service Provider, the prominent segments in contract outsourcing which accounts for lower costs and the specialization that Contract Research Organizations (CROs) offer, including those located within Asia Pacific.
By Type, Spontaneous reporting, with all its limitations is central to pharmacovigilance and leads the way in detecting novel/rare adverse drug reactions.
By Process Flow, signal detection dominated which is the early identification of potential safety concerns based on data from diverse sources.
By Therapeutic Area, oncology space is a rapidly expanding vertical since cancer treatments are complicated and require very comprehensive safety monitoring.
By End Use, includes pharmaceuticals, biotechnology companies, medical device manufacturers, and others.
Pharmacovigilance Market Key Segmentation:
By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
By Process Flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
By End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
Pharmacovigilance: North America and Europe Take the Lead
In 2023, North America held the largest share of pharmacovigilance system status due to a high number of major pharmaceutical companies and which is following strict regulatory guidelines such as REMS (Restriction on sale drugs security); presence for drug-related issues. The country, especially the US is a significant market due to high drug consumption and strict regulatory controls.
Another important region is Europe which has strong regulatory requirements (e.g. EMA RMP) as well an increasing number of clinical trials more less focused on drug development in recent times. The region has experienced increased reporting of adverse drug reactions, which means that careful vigilance is required.
Buy a Single-User PDF of Pharmacovigilance Market Analysis & Outlook Report 2024-2032@ https://www.snsinsider.com/checkout/3095
Recent Developments
USFDA issues no observation to Natco Pharma for Pharmacovigilance
In April 2024, Hyderabad-based Natco Pharma has successfully completed USFDA inspection at its pharmacovigilance -compliance unit with zero observations. With this receipt, the company has achieved an EIR from the USFDA which means there is compliance with good regulatory standards.
Table of Contents – Major Key Points
1. Introduction
2. Industry Flowchart
3. Research Methodology
4. Market Dynamics
5. Porter’s 5 Forces Model
6. Pest Analysis
7. Pharmacovigilance Market Segmentation, By Product Life Cycle
8. Pharmacovigilance Market Segmentation, By Service Provider
9. Pharmacovigilance Market Segmentation, By Type
10. Pharmacovigilance Market Segmentation, By Process Flow
11. Pharmacovigilance Market Segmentation, By Therapeutic Area
12. Pharmacovigilance Market Segmentation, By End Use
13. Regional Analysis
14. Company Profiles
15. Competitive Landscape
16. Use Case and Best Practices
17. Conclusion
Access Complete Report Details of Pharmacovigilance Market Outlook 2024-2032@ https://www.snsinsider.com/reports/pharmacovigilance-market-3095
[For more information or need any customization research mail us at info@snsinsider.com]
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
