New York, USA, July 16, 2024 (GLOBE NEWSWIRE) -- Friedreich's Ataxia Market to Grow at a Substantial Growth Rate by 2034, Examines DelveInsight | Key Companies - Retrotope, PTC Therapeutics, Minoryx Therapeutics, Biogen, Lexeo Therapeutics, Larimar Therapeutics
The dynamics of Friedreich's ataxia market are anticipated to change as companies across the globe are thoroughly working toward the development of new drug therapy options to treat this disease.
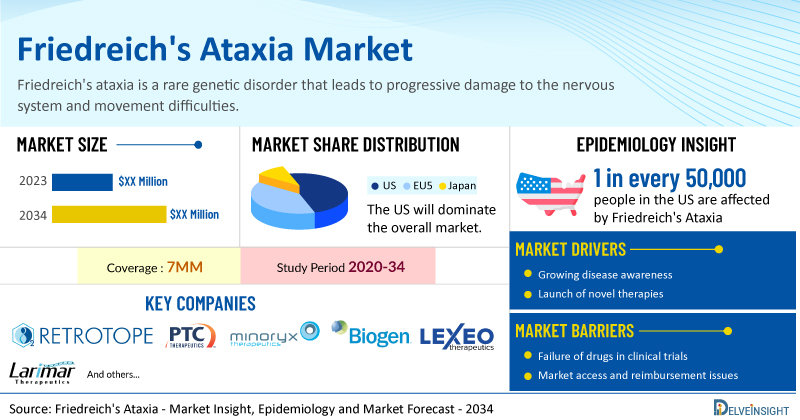
DelveInsight’s Friedreich's Ataxia Market Insights report includes a comprehensive understanding of current treatment practices, Friedreich's ataxia emerging drugs, market share of individual therapies, and current and forecasted Friedreich's ataxia market size from 2020 to 2034, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Friedreich's Ataxia Market Report
- According to DelveInsight’s analysis, the market size of Friedreich's ataxia in the 7MM is expected to grow at a significant CAGR by 2034.
- Friedreich's ataxia is the most common form of hereditary ataxia in the United States, affecting about 1 in every 50,000 people.
- Prominent companies working in the domain of Friedreich's ataxia, including Retrotope, PTC Therapeutics, Minoryx Therapeutics, Biogen, Lexeo Therapeutics, Larimar Therapeutics, Inc., and others, are actively working on innovative drugs for Friedreich's ataxia. These novel Friedreich's ataxia therapies are anticipated to enter the Friedreich's ataxia market in the forecast period and are expected to change the market.
- Some of the key therapies for Friedreich's ataxia treatment include RT001, Vatiquinone, Leriglitazone, Omaveloxolone, LX2006, CTI-1601, and others.
Discover which therapies are expected to grab Friedreich's ataxia market share @ Friedreich's Ataxia Market Report
Friedreich's Ataxia Overview
Friedreich's ataxia is a rare genetic disorder that leads to progressive damage to the nervous system and movement difficulties. This condition typically starts in childhood, resulting in worsening muscle coordination over time. It arises from a mutation in the FXN gene, which encodes the protein frataxin. Individuals who inherit two defective copies of this gene, one from each parent, will develop the disease. The disorder is divided into two subtypes: LOFA, with onset between ages 26 and 39, and VLOFA, with onset after age 40.
Symptoms of Friedreich's ataxia usually start between the ages of 5 and 15. The first neurological signs are often trouble walking and maintaining balance. Early indicators also include slow and slurred speech, which eventually becomes hesitant and jerky, a condition known as "scanning of speech." As the disease progresses, coordination issues (ataxia) can impact all muscles, gradually worsening and spreading to the arms and torso.
Diagnosing Friedreich's ataxia involves a thorough clinical examination, including a detailed physical check for balance issues, loss of joint sensation (proprioception), absence of reflexes, and other neurological symptoms. Genetic testing now offers a definitive diagnosis.
Friedreich's Ataxia Epidemiology Segmentation
The Friedreich's ataxia epidemiology section provides insights into the historical and current Friedreich's ataxia patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The Friedreich's ataxia market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Prevalent Cases of Friedreich’s Ataxia
- Prevalent Cases of Friedreich’s Ataxia Based on Onset Types
- Diagnosed and Treatable Cases of Friedreich’s Ataxia
Download the report to understand which factors are driving Friedreich's ataxia epidemiology trends @ Friedreich's Ataxia Epidemiological Insights
Friedreich's Ataxia Treatment Market
Current treatments for Friedreich's ataxia focus on alleviating specific symptoms rather than addressing the root cause. These symptom-targeted therapies remain the cornerstone of Friedreich's ataxia care. Although there is no cure, various management strategies can enhance the quality of life for those affected by the disease. Thankfully, heart disease, the most life-threatening symptom of Friedreich's ataxia, can be managed with treatments commonly used for the general population.
Certain medications, including ACE inhibitors, diuretics, and beta-blockers, reduce the heart's workload, while pacemakers or other medications can stabilize an irregular heartbeat. Similarly, insulin can help manage diabetes in patients with Friedreich's ataxia. Additionally, surgical interventions are sometimes necessary to correct foot deformities and scoliosis, and although these procedures are significant, they are typically effective.
Although no treatment regimen can halt the progression of ataxia or muscle weakness in Friedreich's ataxia, rehabilitation therapy is typically seen as helpful in managing these issues. Additionally, physical therapy can aid in stretching tight muscles and increasing flexibility, while speech therapy can assist in retraining tongue and facial muscles to enhance speech and swallowing.
In February 2023, the FDA approved Reata Pharmaceuticals for omaveloxolone, marketed as SKYCLARYS, to treat Friedreich’s ataxia in individuals aged 16 and older. More recently, in February 2024, the European Commission also authorized Biogen’s SKYCLARYS (omaveloxolone) for treating Friedreich's ataxia in the same age group.
Learn more about the FDA-approved drugs for Friedreich's ataxia @ Drugs for Friedreich's Ataxia Treatment
Friedreich's Ataxia Emerging Drugs and Companies
Key products such as RT001 (Retrotope), Vatiquinone (PTC Therapeutics), and Leriglitazone (Minoryx Therapeutics) might influence the market size during the forecast period (2024–2034).
RT001, developed by Retrotope, is a modified form of ethyl linoleate that hinders lipid peroxidation, potentially diminishing cellular harm and restoring mitochondrial function. In February 2021, the FDA in the United States expedited its review process, granting fast-track status for treating Friedreich's ataxia. It had previously received orphan drug designation for this condition in 2016. By August 2021, Retrotope had finished a combined Phase II/III trial, evaluating RT001's safety and effectiveness in individuals with Friedreich's ataxia.
Vatiquinone, developed by PTC Therapeutics, is a potential treatment compound that hampers 15-Lipoxygenase, an enzyme pivotal in regulating pathways associated with oxidative stress and inflammation, both crucial aspects in neurological disease progression. In March 2014, the FDA expedited Vatiquinone's development by granting it fast-track designation for addressing Friedreich's Ataxia. Additionally, it has obtained orphan drug status from the FDA for the same condition. Currently, Vatiquinone is undergoing Phase III clinical trials to evaluate its efficacy in treating Friedreich's Ataxia.
The other therapies in the pipeline for Friedreich's ataxia treatment include
- Omaveloxolone: Biogen
- LX2006: Lexeo Therapeutics
- CTI-1601: Larimar Therapeutics, Inc.
The anticipated launch of these emerging therapies are poised to transform Friedreich's ataxia market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape Friedreich's ataxia market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about Friedreich's ataxia clinical trials, visit @ Friedreich's Ataxia Treatment Drugs
Friedreich's Ataxia Market Dynamics
Friedreich's ataxia market dynamics are anticipated to change in the coming years. One of the key drivers is the increasing prevalence of the disease and the urgent need for effective treatments. Advancements in genetic research and biotechnology have spurred the development of novel therapeutic approaches, including gene therapy and disease-modifying treatments. Furthermore, growing awareness among healthcare professionals and patients, combined with robust support from governmental and non-governmental organizations for rare disease research, has significantly contributed to market growth. The rise in funding for clinical trials and research initiatives aimed at understanding the disease's pathophysiology better and developing targeted therapies further fuels the Friedreich's ataxia market's expansion.
Furthermore, many potential therapies are being investigated for the treatment of Friedreich's ataxia, and it is safe to predict that the treatment space will significantly impact Friedreich's ataxia market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of Friedreich's ataxia market in the 7MM.
However, several factors may impede the growth of the Friedreich's ataxia market. The complex interplay of genetic factors and the variability in symptom presentation complicate the development of targeted therapies, thus discouraging pharmaceutical investment. Additionally, challenges in conducting clinical trials, such as patient recruitment and endpoint selection, hinder therapeutic advancement. Moreover, reimbursement hurdles and the high cost of research and development further impede the commercial viability of potential treatments, creating substantial barriers to addressing the unmet medical needs of Friedreich's ataxia patients.
Moreover, Friedreich's ataxia treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, Friedreich's ataxia market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact Friedreich's ataxia market growth.
| Friedreich's Ataxia Report Metrics | Details |
| Study Period | 2020–2034 |
| Friedreich's Ataxia Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Friedreich's Ataxia Companies | Retrotope, PTC Therapeutics, Minoryx Therapeutics, Biogen, Lexeo Therapeutics, Larimar Therapeutics, Inc., and others |
| Key Friedreich's Ataxia Therapies | RT001, Vatiquinone, Leriglitazone, Omaveloxolone, LX2006, CTI-1601, and others |
Scope of the Friedreich's Ataxia Market Report
- Friedreich's Ataxia Therapeutic Assessment: Friedreich's Ataxia current marketed and emerging therapies
- Friedreich's Ataxia Market Dynamics: Attribute Analysis of Emerging Friedreich's Ataxia Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Friedreich's Ataxia Market Access and Reimbursement
Discover more about Friedreich's ataxia drugs in development @ Friedreich's Ataxia Clinical Trials
Table of Contents
| 1. | Friedreich's Ataxia Market Key Insights |
| 2. | Friedreich's Ataxia Market Report Introduction |
| 3. | Friedreich's Ataxia Market Overview at a Glance |
| 4. | Friedreich's Ataxia Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Friedreich's Ataxia Treatment and Management |
| 7. | Friedreich's Ataxia Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Friedreich's Ataxia Marketed Drugs |
| 10. | Friedreich's Ataxia Emerging Drugs |
| 11. | Seven Major Friedreich's Ataxia Market Analysis |
| 12. | Friedreich's Ataxia Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Friedreich's Ataxia Epidemiology Forecast
Friedreich's Ataxia Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted Friedreich's ataxia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Friedreich's Ataxia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Friedreich's ataxia companies, including PTC Therapeutics, Retrotope, Reata Pharmaceuticals, Minoryx Therapeutics, Larimar Therapeutics, LEXEO Therapeutics, Exicure, StrideBio, Voyager Therapeutics, Lacerta Therapeutics, among others.
Ataxia Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key ataxia companies, including AstraZeneca, Merck Sharp & Dohme LLC, Bayer, Roche, among others.
Ataxia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key ataxia companies, including Retrotope, Reata Pharmaceuticals, PTC Therapeutics, Metro International Biotech, LLC, Design Therapeutics, Larimar Therapeutics, Minoryx Therapeutics, EryDel, Biogen, Matrix Biomed, IntraBio, Biohaven Pharmaceuticals, Inc., Stealth BioTherapeutics Inc., Acasti Pharma, Seelos Therapeutics, Kissei Pharmaceutical, Vico Therapeutics, Q-State Biosciences, Locanabio, Lexeo Therapeutics, Voyager Therapeutics, CRISPR Therapeutics, Capsida Biotherapeutics, AavantiBio, StrideBio, Wave Life Sciences, REPROCELL, SHIONOGI & Co., CORESTEM, Blade Therapeutics, Exicure, Lacerta Therapeutics, Healx Ltd., Uniqure, Ionis Pharmaceuticals, Jupiter Neurosciences, among others.
Ataxia Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted ataxia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
