Pune, Aug. 01, 2024 (GLOBE NEWSWIRE) -- Medical Device Reprocessing Market Analysis:
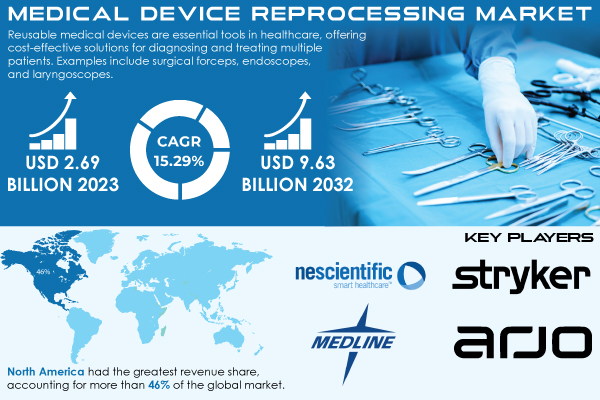
“According to SNS Insider Research, The Medical Device Reprocessing Market size was estimated at US$ 2.69 billion in 2023 and is expected to reach US$ 9.63 billion by 2032 & grow at a CAGR of 15.29% during the forecast period of 2024-2032.”
Reusable medical devices are robust components of healthcare, providing an economical solution for the diagnosis and treatment patient after patients. Such instruments include surgical forceps, endoscopes and laryngoscopes. But these can’t be used without reprocessing to remove contaminants and infections. Although the likelihood of disease transmission via improperly processed devices is small, the impact on public health from outbreaks can still be substantial. Comprehensive disinfection and sterilization of medical equipment are paramount, particularly in the light of preventing communicable diseases when these devices are used on patients according to recent statement issued by Centers for Disease Control and Prevention (CDC) utilising the industry standards and best practices in reprocessing medical devices greatly reduces infection risk, improving patient safety across all healthcare facilities.
Supporting the demand for these devices is high in market penetration owing to growth in surgical procedures and rising requirements of reliable, durable equipment. The sheer number of surgeries and the reprocessing capacity required which are reflective of a significant role for medical device reprocessing. Between 2019 and2020, more than 13 million surgeries were performed worldwide, with women experiencing at least half of them. This large scale indicates an utmost importance for proper, safe and quality medical device reprocessing to protect patients from infection during these procedures maintaining low potential risks. Surgical instruments, endoscopy equipment and respiratory care devices are among significant segments. Regardless, these components might suffer availability issues due to challenges in the supply chain like material shortages and manufacturing complications. The need for strict sterilization protocols and risk of contamination contributes to the price of these devices, which in turn inhibits these devices from ever reaching widespread use and due to cost affective and affordability this hampers certain healthcare facilities.
Get a Sample Report of Medical Device Reprocessing Market@ https://www.snsinsider.com/sample-request/3485
Major Players Analysis Listed in this Report are:
- Stryker
- Innovative Health
- NEScientific, Inc.
- Medline Industries, LP
- Arjo
- Vanguard AG
- Cardinal Health
- SureTek Medical
- Soma Tech Intl
- Johnson & Johnson MedTech
- Other Players
Reprocessing of medical devices is a routine part of practice needed for patient and staff safety, and compliance to do so when the device or instrument can be used again. For the healthcare facility, reprocessing a device is economically beneficial because it avoids purchasing disposable instruments or devices that cost per use and are disposed of after one patient procedure. Though other costs are related to medical device reprocessing - repair and replacement of devices, cleaning chemistries as well as capital purchases such tap sinks, washer/disinfectors sterilizers etc., third-party or hospital-based reprocessing also provide some financial benefits for the facility. Additionally, the handling of medical waste is a complex manner in terms of containment and treatment as well under OSHA guidelines for disposal practices within US.
Although the chance for transmission of an infection from a medical device that is not ideally reprocessed is small compared to total use, there are many devices in service and even rare outbreaks caused by such failures receive widespread public attention. Regrettably, most of these infections are never detected or reported to the FDA. Thus, the actual number of reprocessing-related healthcare-associated infections remains unknown.
Medical Device Reprocessing Market Report Scope:
| Report Attributes | Details |
| Market Size in 2023 | US$ 2.69 Bn |
| Market Size by 2032 | US$ 9.63 Bn |
| CAGR | CAGR of 15.29% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Key Regional Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]). Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
| Key Growth Drivers | • Supply chain cost savings in healthcare facilities are increasing. |
Need any customization research or industry insights on Medical Device Reprocessing Market, Enquire Now@ https://www.snsinsider.com/enquiry/3485
In the case of reprocessed devices, FDA is currently working on solutions to limitations related implementation intended mitigate potential infection risk. Building on their robust oversight of medical devices, they set forth clear requirements for the marketplace, raise manufacturing standards for improved patient safety and work with manufacturers to address new public health issues. FDA will also behind raising awareness, convening the healthcare stakeholders and spurring device redesign to help reduce recurrence of this type of event.
While the potential advantages to reprocessing medical devices go beyond just cost and environmental benefits, significant obstacles exist in creating standard procedures for properly carrying out these processes. Compliance with strict guidelines given by such organizations as the Association for Advanced Medical Instrument (AAMI) is necessary to avoid hazardous situations and maintain patient safety. Every step of the reprocessing cycle requires careful attention to ensure that healthcare-associated infections do not occur. Creating an effective reprocessing program that meets hospital policies, industry best practices as well as the original equipment manufacturer guidelines is crucial to achieving optimal patient outcomes while altering all of these complexities. Furthermore, despite the challenges in place, the medical devices reprocessing market expected to grow at high rate over the forecast period.
Medical Device Reprocessing Market Key Segmentation:
By Type
- Reprocessing Support & Services
- Reprocessed Medical Devices
By Device Category
- Critical Devices
- Semi-Critical Devices
- Non-Critical Devices
By Application
- Cardiology
- Gastroenterology
- Gynecology
- Arthroscopy & Orthopedic Surgery
- General Surgery and Anesthesia
- Other Device Categories (Urology, non-invasive surgeries, patient monitoring)
Medical Device Reprocessing Market Is Mainly Driven by The Cost Reduction with The Increasing Prevalence of Chronic Disease
- The idea of reprocessing medical devices is quite appealing to healthcare facilities as it represents significant cost savings versus buying new, which can in turn be passed down to the patient.
- As environmental concerns have grown among the healthcare field, sustainability targets become table stakes for operational success and use of reprocessed medical products help in meeting those standards.
- The increasing prevalence of chronic diseases is requiring an increased number of medical procedures, which in turn fuels the growth for both new and reprocessed devices.
- Rising use of reprocessing devices due to enhancement in the reprocessing technologies which is safe and effective further increases the market confidence.
- Reprocessed devices are adopting by healthcare providers but only in the presence of supportive regulatory frameworks.
Buy an Enterprise-User PDF of Medical Device Reprocessing Market Analysis & Outlook Report 2024-2032@ https://www.snsinsider.com/checkout/3485
Medical Device Reprocessing Market Is Restraint by Lack of Awareness as Well As Due to Limited Device Compatibility
- The threat of infections from inadequately reprocessed devices can dampen market prospects.
- Low awareness of reprocessed device benefits and safety among healthcare professionals may hinder adoption.
- It can add to the operational cost of reprocessing facilities if they need to comply with strict norms.
- Market potential is limited due to unsuitableness of reprocessing for many medical devices.
- The buildout of reprocessing facilities can be capital-intensive and thus not feasible for all users.
Table of Contents – Major Key Points
1. Introduction
2. Industry Flowchart
3. Research Methodology
4. Market Dynamics
5. Porter’s 5 Forces Model
6. Pest Analysis
7. Medical Device Reprocessing Market Segmentation, by Type
8. Medical Device Reprocessing Market Segmentation, by Device Category
9. Medical Device Reprocessing Market Segmentation, by Application
10. Regional Analysis
11. Company Profiles
12. Competitive Landscape
12.1 Competitive Benchmarking
12.2 Market Share Analysis
12.3 Recent Developments
12.3.1 Industry News
12.3.2 Company News
12.3.3 Mergers & Acquisitions
13. Use Case and Best Practices
14. Conclusion
Access Complete Report Details of Medical Device Reprocessing Market Analysis Report 2024-2032@ https://www.snsinsider.com/reports/medical-device-reprocessing-market-3485
[For more information or need any customization research mail us at info@snsinsider.com]
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
