New York, USA, Aug. 13, 2024 (GLOBE NEWSWIRE) -- Prader–Willi Syndrome Market is Expected to Showcase Growth at a CAGR of ~6% by 2034 | DelveInsight
The Prader–Willi syndrome market is experiencing growth due to increasing in diagnosis rate owing to an increase in genetic testing, more and more identification of other lesser-known genetic types including translocation, disomy, and imprinting defects, and the expected launch of Soleno’s DCCR in 2025, first therapy which targets hyperphagia in PWS patients.
DelveInsight’s Prader–Willi Syndrome Market Insights report includes a comprehensive understanding of current treatment practices, emerging Prader–Willi syndrome drugs, market share of individual therapies, and current and forecasted Prader–Willi syndrome market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Prader–Willi Syndrome Market Report
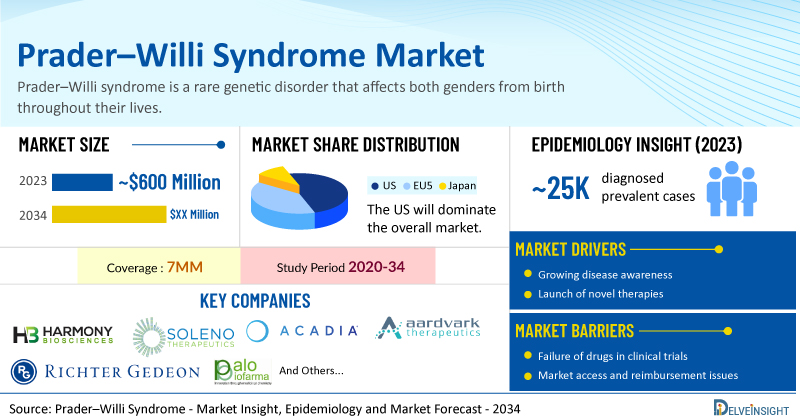
- According to DelveInsight’s analysis, the market size of Prader–Willi syndrome in the 7MM reached USD 600 million in 2023 and is expected to increase by 2034, majorly due to the expected drastic YoY growth in diagnosis rate, and the high cost of the expected launch of several key therapies
- Based on DelveInsight's assessment in 2023, the 7MM had 24,990 diagnosed prevalent cases of Prader–Willi syndrome. Even though multiple literature suggest a higher average diagnosis rate within a range of 65-80%, however when compared based on real-world evidence (through registries) the diagnosis gap seems to be slightly larger. Even though currently the diagnosis gap remains larger, these cases are expected to rise due to the advancements in diagnostic capabilities, awareness about the diseases, and the setting up of more PWS registries during the forecast period (2024−2034).
- The estimated coverage of PWS patients in country-specific registries is limited. As a result, even when strict criteria are applied during patient recruitment for these registries, they do not provide an accurate representation of the actual diagnosed cases.
- In the US, roughly 70% of PWS patients are children whereas in Japan child population contributes to roughly 55% only.
- In 2020, the highest number of cases of PWS was in the age group 5-11 years, i.e., 2,972 cases and the lowest number of cases was in the age group 40+ years, i.e., 942 cases in the US.
- Prominent companies working in the domain of Prader–Willi syndrome, including Harmony Biosciences, Soleno Therapeutics, Acadia Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, Saniona, and others, are actively working on innovative Prader–Willi syndrome drugs. These novel Prader–Willi syndrome therapies are anticipated to enter the Prader–Willi syndrome market in the forecast period and are expected to change the market.
- Some of the key Prader–Willi syndrome treatments include WAKIX (pitolisant), Diazoxide Choline Controlled-Release (DCCR), Carbetocin (ACP-101, LV-101), ARD-101, RGH-706, PBF-999, Tesomet, and others.
- DCCR is expected to receive the first mover advantage in 2025 in the US with the highest commercial opportunity owing to its established efficacy in pivotal trials. On the other hand, Acadia Pharma's Carbetocin with a failed primary endpoint, lowers our expectation for probability of success. Harmony Biosciences' WAKIX (pitolisant) is the only therapy being evaluated for PWS patients with Excessive Daytime Sleepiness (EDS), which is roughly 50% of the total PWS patients.
- There is high uncertainty around both Gedeon Richter and Palobiofarma's therapies due to the unavailability of substantial evidence around safety & efficacy data. Meanwhile, Palobiofarma's PBF-999 first launch is expected in Spain.
Discover which therapies are expected to grab the Prader–Willi syndrome market share @ Prader–Willi Syndrome Market Report
Prader–Willi Syndrome Overview
Prader–Willi syndrome is a rare genetic disorder that affects both genders from birth throughout their lives. It is characterized by low muscle tone, motor development delays, mild to moderate learning difficulties, incomplete sexual development, and emotional and social immaturity, which can lead to challenging behaviors. A persistent, insatiable appetite often develops in childhood, resulting in food-seeking behaviors, stealing, and potentially dangerous obesity if not managed with strict food control and exercise.
Diagnosing Prader–Willi syndrome usually starts with parents noticing early signs such as poor muscle tone and feeding problems in infancy. Concerned parents then consult a pediatrician, who, noticing developmental delays and distinctive facial features, refers them to a genetic specialist. The specialist performs a thorough clinical examination and orders genetic tests, primarily DNA methylation analysis, to confirm the diagnosis.
Additional tests like FISH and MS-MLPA may be used if needed. After diagnosis, the specialist explains the condition and develops a multidisciplinary care plan involving endocrinologists, dietitians, and therapists. Regular follow-ups and interventions are crucial for managing symptoms and supporting the child's development.
Prader–Willi Syndrome Epidemiology Segmentation
The Prader–Willi syndrome epidemiology section provides insights into the historical and current Prader–Willi syndrome patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The Prader–Willi syndrome market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Prevalent Cases of Prader-Willi Syndrome
- Diagnosed Prevalent Cases of Prader-Willi Syndrome
- Mutation-specific Diagnosed Prevalent Cases of Prader-Willi Syndrome
- Treated Cases of Prader-Willi Syndrome
Download the report to understand which factors are driving Prader–Willi syndrome epidemiology trends @ Prader–Willi Syndrome Epidemiological Insights
Prader–Willi Syndrome Treatment Market
Current treatment options for Prader–Willi syndrome are limited and mainly involve lifestyle changes to prevent obesity-related fatalities. Nearly half of the deaths in Prader–Willi syndrome patients under 18 are associated with food-seeking behaviors, including choking and accidents. Managing Prader–Willi syndrome involves various therapeutic areas—nutritional, developmental, educational, hormonal, and behavioral—with each stage of development needing specific strategies.
Growth hormone therapy has been found to increase growth velocity and height, improve body composition, and, with appropriate dietary management, prevent obesity. It also boosts physical and respiratory performance, enhancing overall quality of life and potentially reducing long-term cardiovascular and metabolic risks like hypercholesterolemia and diabetes. While there is no cure for Prader–Willi syndrome and it cannot be prevented, specialized healthcare can improve a child's quality of life and address some of the associated challenges.
The FDA has approved three growth hormone products for treating Prader–Willi syndrome: GENOTROPIN from Pfizer, NORDITROPIN from Novo Nordisk, and OMNITROPE from Sandoz. This authorization allows doctors to prescribe these treatments as alternatives. The selection of a growth hormone often depends on factors such as the doctor’s expertise, insurance coverage, cost, ease of use, and the patient's medical history or sensitivities.
In Europe, the market is similar to that in the US, with growth hormone and its synthetic versions being commonly used, particularly for children and adolescents. The EMA approved OMNITROPE as its first biosimilar growth hormone in 2006, and GENOTROPIN is also a key-approved product. In March 2012, the EMA’s Committee for Medicinal Products for Human Use (CHMP) concluded an arbitration process, rejecting a proposal to include a new indication for NORDITROPIN for use in children with Prader–Willi syndrome.
Learn more about the market of Prader–Willi syndrome @ Prader–Willi Syndrome Treatment
Prader–Willi Syndrome Emerging Drugs and Companies
Prader–Willi syndrome pipeline possesses some drugs in mid and late-stage developments to be approved soon. The emerging landscape holds a diverse range of therapeutic alternatives for treatment, including H3 Histamine receptor inverse agonists/antagonists (WAKIX [pitolisant]), KATP channel activators (Potassium channel agonists) (DCCR), Oxytocin receptor agonists (Carbetocin [LV-101]), Targeting extra-oral bitter taste receptors (TAS2R) (ARD-101), and others. The expected launch of these shall further create a positive impact on the market.
WAKIX (pitolisant) is a targeted antagonist/inverse agonist of histamine 3 (H₃) receptors. It is approved by the FDA for treating excessive daytime sleepiness or cataplexy in adults with narcolepsy. Currently, pitolisant is not approved for patients with Prader–Willi syndrome but is being studied for this condition. Harmony Biosciences' WAKIX is the sole treatment under investigation for Prader–Willi syndrome patients experiencing EDS, which affects about 50% of individuals with Prader–Willi syndrome. We anticipate that WAKIX will see prolonged market success and strong commercial uptake due to the significant unmet need in this patient group, limited competition, and its proven effectiveness in treating narcolepsy.
DCCR is a new, proprietary extended-release form of diazoxide choline, the crystalline salt of diazoxide, which is taken once daily. While diazoxide itself has been used for many years to treat a few rare diseases in neonates, infants, children, and adults, it is not approved for Prader-Willi Syndrome (PWS). DCCR was tested in a Phase III trial (C601 or DESTINY PWS), a 13-week randomized, double-blind, placebo-controlled study that completed enrollment in January 2020, involving 127 participants across 29 sites in the US and UK.
The anticipated launch of these emerging therapies are poised to transform the Prader–Willi syndrome market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the Prader–Willi syndrome market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about Prader–Willi syndrome clinical trials, visit @ Prader–Willi Syndrome Treatment Drugs
Prader–Willi Syndrome Market Dynamics
The Prader–Willi syndrome market dynamics are anticipated to change in the coming years.
Furthermore, many potential therapies are being investigated for the treatment of Prader–Willi syndrome, and it is safe to predict that the treatment space will significantly impact the Prader–Willi syndrome market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the Prader–Willi syndrome market in the 7MM.
However, several factors may impede the growth of the Prader–Willi syndrome market.
Moreover, Prader–Willi syndrome treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the Prader–Willi syndrome market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the Prader–Willi syndrome market growth.
| Prader–Willi Syndrome Report Metrics | Details |
| Study Period | 2020–2034 |
| Prader–Willi Syndrome Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Prader–Willi Syndrome Market CAGR | Approx. 6% |
| Prader–Willi Syndrome Market Size in 2023 | USD 600 Million |
| Key Prader–Willi Syndrome Companies | Harmony Biosciences, Soleno Therapeutics, Acadia Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, Saniona, Novo Nordisk, and others |
| Key Prader–Willi Syndrome | WAKIX (pitolisant), Diazoxide Choline Controlled-Release (DCCR), Carbetocin (ACP-101, LV-101), ARD-101, RGH-706, PBF-999, Tesomet, NORDITROPIN, and others |
Scope of the Prader–Willi Syndrome Market Report
- Prader–Willi Syndrome Therapeutic Assessment: Prader–Willi Syndrome current marketed and emerging therapies
- Prader–Willi Syndrome Market Dynamics: Conjoint Analysis of Emerging Prader–Willi Syndrome Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Pricing Analysis, Market Access and Reimbursement
Discover more about Prader–Willi syndrome in development @ Prader–Willi Syndrome Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | Executive Summary |
| 4 | PWS Market Overview at a Glance |
| 4.1 | Market Share by Therapies (%) Distribution of PWS in 2020 in the 7MM |
| 4.2 | Market Share by Therapies (%) Distribution of PWS in 2034 in the 7MM |
| 5 | Key Events |
| 6 | Epidemiology and Market Forecast Methodology |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Signs and Symptoms |
| 7.3 | Causes |
| 7.4 | Complications |
| 7.5 | Stages of PWS |
| 7.6 | Diagnosis |
| 7.7 | Differential Diagnosis |
| 8 | Treatment and Management |
| 8.1 | Treatment Guidelines |
| 8.1.1 | PWS: Screening Guidance for Children and Transition into Adulthood (Appraisal of Guidelines Research and Evaluation Instrument II and 2020 RCPCH Standards for Development of Pediatric Guideline, 2024) |
| 8.1.2 | Diagnosis and Management Guidelines of Sleep Disorders in PWS (Journal of Clinical Sleep Medicine, 2022) |
| 8.1.3 | NHS Shropshire Community Health Management Guidelines (2020) |
| 8.1.4 | Update of the European Molecular Genetics Quality Network (EMQN)/ Association for Clinical Genomic Science (ACGS) Best Practice Guideline for Molecular Analysis of PWS (2019) |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationales |
| 9.3 | Total Prevalent Cases of PWS in the 7MM |
| 9.4 | Total Diagnosed Prevalent Cases of PWS in the 7MM |
| 9.5 | The United States |
| 9.5.1 | Total Prevalent Cases of PWS in the US |
| 9.5.2 | Total Diagnosed Prevalent Cases of PWS in the United States |
| 9.5.3 | Age-specific Cases of PWS in the United States |
| 9.5.4 | PWS Cases by Genetic Subtype in the US |
| 9.6 | EU4 and the UK |
| 9.6.1 | Total Prevalent Cases of PWS in EU4 and the UK |
| 9.6.2 | Total Diagnosed Prevalent Cases of PWS in EU4 and the UK |
| 9.6.3 | Age-specific Cases of PWS in EU4 and the UK |
| 9.6.4 | PWS Cases by Genetic Subtype in EU4 and the UK |
| 9.7 | Japan |
| 9.7.1 | Total Prevalent Cases of PWS in Japan |
| 9.7.2 | Total Diagnosed Prevalent Cases of PWS in Japan |
| 9.7.3 | Age-specific Cases of PWS in Japan |
| 9.7.4 | PWS Cases by Genetic Subtype in Japan |
| 10 | Patient Journey |
| 11 | Marketed Drugs |
| 11.1 | Key Competitors |
| 11.2 | NORDITROPIN: Novo Nordisk |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestone |
| 11.2.3 | Safety and Efficacy |
| 11.2.4 | Product Profile |
| 12 | Emerging Drugs |
| 12.1 | Key Competitors |
| 12.2 | WAKIX (pitolisant): Harmony Biosciences |
| 12.2.1 | Product Description |
| 12.2.2 | Other Developmental Activities |
| 12.2.3 | Clinical Development |
| 12.2.3.1 | Clinical Trial Information |
| 12.2.4 | Safety and Efficacy |
| 12.3 | Diazoxide Choline Controlled-Release (DCCR): Soleno Therapeutics |
| 12.3.1 | Product Description |
| 12.3.2 | Other Developmental Activities |
| 12.3.3 | Clinical Development |
| 12.3.3.1 | Clinical Trial Information |
| 12.3.4 | Safety and Efficacy |
| 12.4 | Carbetocin (ACP-101, LV-101): Acadia Pharmaceuticals |
| 12.4.1 | Product Description |
| 12.4.2 | Other Developmental Activities |
| 12.4.3 | Clinical Development |
| 12.4.3.1 | Clinical Trial Information |
| 12.4.4 | Safety and Efficacy |
| 12.5 | ARD-101: Aardvark Therapeutics |
| 12.5.1 | Product Description |
| 12.5.2 | Other Developmental Activities |
| 12.5.3 | Clinical Development |
| 12.5.3.1 | Clinical Trial Information |
| 12.5.4 | Safety and Efficacy |
| 12.6 | RGH-706: Gedeon Richter |
| 12.6.1 | Product Description |
| 12.6.2 | Clinical Development |
| 12.6.2.1 | Clinical Trial Information |
| 12.7 | PBF-999: Palobiofarma |
| 12.7.1 | Product Description |
| 12.7.2 | Other Developmental Activities |
| 12.7.3 | Clinical Development |
| 12.7.3.1 | Clinical Trial Information |
| 12.8 | Tesomet: Saniona |
| 12.8.1 | Product Description |
| 12.8.2 | Other Developmental Activities |
| 12.8.3 | Clinical Development |
| 12.8.3.1 | Clinical Trial Information |
| 12.8.4 | Safety and Efficacy |
| 13 | PWS: Market Analysis |
| 13.1 | Key Findings |
| 13.2 | Market outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Market Forecast Assumptions |
| 13.4.1 | Cost Assumptions and Rebates |
| 13.4.2 | Pricing Trends |
| 13.4.3 | Analogue Assessment |
| 13.4.4 | Launch Year and Therapy Uptakes |
| 13.5 | Total Market Size of PWS in the 7MM |
| 13.6 | The United States Market Size |
| 13.6.1 | Total Market Size of PWS in the United States |
| 13.6.2 | Market Size of PWS by Therapies in the United States |
| 13.7 | EU4 AND THE UK MARKET SIZE |
| 13.7.1 | Total Market Size of PWS in EU4 and the UK |
| 13.7.2 | Market Size of PWS by Therapies in EU4 and the UK |
| 13.8 | Japan Market Size |
| 13.8.1 | Total Market Size of PWS in Japan |
| 13.8.2 | Market Size of PWS by Therapies in Japan |
| 14 | Unmet Needs |
| 15 | SWOT Analysis |
| 16 | KOL Views |
| 17 | Market Access and Reimbursement |
| 17.1 | United States |
| 17.1.1 | Centre for Medicare and Medicaid Services (CMS) |
| 17.2 | EU4 and the UK |
| 17.2.1 | Germany |
| 17.2.2 | France |
| 17.2.3 | Italy |
| 17.2.4 | Spain |
| 17.2.5 | United Kingdom |
| 17.3 | Japan |
| 17.3.1 | MHLW |
| 17.4 | Market Access and Reimbursement of PWS |
| 18 | Appendix |
| 18.1 | Bibliography |
| 18.2 | Report Methodology |
| 19 | DelveInsight Capabilities |
| 20 | Disclaimer |
| 21 | About DelveInsight |
Related Reports
Prader–Willi Syndrome Epidemiology Forecast
Prader–Willi Syndrome Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted Prader–Willi syndrome epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Prader–Willi Syndrome Pipeline
Prader–Willi Syndrome Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Prader–Willi syndrome companies, including Soleno Therapeutics, Levo Therapeutics, Inversago Pharma, Saniona, LG Life Sciences, GLWL Research, OptiNose, Larimar Therapeutics, Helsinn, ConSynance Therapeutics, Neuren Pharmaceuticals, Radius Health, Rhythm, Tonix Pharmaceuticals, among others.
Obesity Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key obesity companies, including Novo Nordisk, Eli Lilly and Company, CSPC Baike (Shandong) Biopharmaceutical, Jiangsu Hengrui Medicine, Carmot Therapeutics, MedImmune, Boehringer Ingelheim, Raziel Therapeutics, Pfizer, Sciwind Biosciences, Empros Pharma, Amgen, Epitomee Medical, ERX Pharmaceuticals, Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, Glaceum, Shionogi, Aardvark Therapeutics, NuSirt Biopharma, Novartis, among others.
Obesity Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key obesity companies, including Zealand Pharma, Sciwind Biosciences, Genexine, Sirnaomics, Sparrow Pharmaceuticals, Shionogi, Regor Pharmaceuticals, Innovent Biologics, Fractyl Health, Pfizer, NodThera Limited, Boehringer Ingelheim, Fractyl Health, TransThera, Clearmind Medicine, PegBio, Biolingus, Eli Lily &Company, Boehringer Ingelheim, Aardvark Therapeutics, Rivus Pharmaceuticals, ERX Pharmaceuticals, BioRestorative Therapies, Amolyt Pharma, Biolexis Therapeutics, Immunwork, among others.
Hypothalamic Obesity Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key hypothalamic obesity companies, including Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, among others.
Hypothalamic Obesity Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products and the key hypothalamic obesity companies, including Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
