Rockville, MD, Sept. 11, 2024 (GLOBE NEWSWIRE) -- According to Fact.MR, a market research and competitive intelligence provider, the global celiac disease diagnostics market is estimated to reach a valuation of US$ 626.2 million in 2024 and is expected to grow at a CAGR of 8.5% during the forecast period of (2024 to 2034).
More accessible, efficient, and accurate testing results from advancements in diagnostic technology, which impacts the growth of the celiac disease diagnostic market. Newer serological diagnostics, like improved lateral flow assays and complex ELISA assays, offer higher sensitivity and specificity in detecting antibodies associated with celiac disease. Fewer false positives and negatives occur as a result. Modern serological techniques allow for the simultaneous detection of many antibodies, such as tTG-IgA, DGP-IgA, and DGP-IgG, providing a comprehensive diagnostic profile from a single sample.
Identifying HLA-DQ2 and HLA-DQ8 genotypes that are associated with higher predisposition to celiac disease has become fast and precise due to advancements in technology. This results in early detection and enhanced risk assessment capabilities providing means for optimized diagnosis. NGS serves to expand knowledge on genetic susceptibility and provides better diagnosis through its detailed genomic information that reveals infrequent mutations associated with coeliac disease. New point-of-care testing kits make it easier to examine patients and speed up decision-making in clinical or home settings by providing rapid results.
For More Insights into the Market, Request a Sample of this Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=10338
Key Takeaways from Market Study:
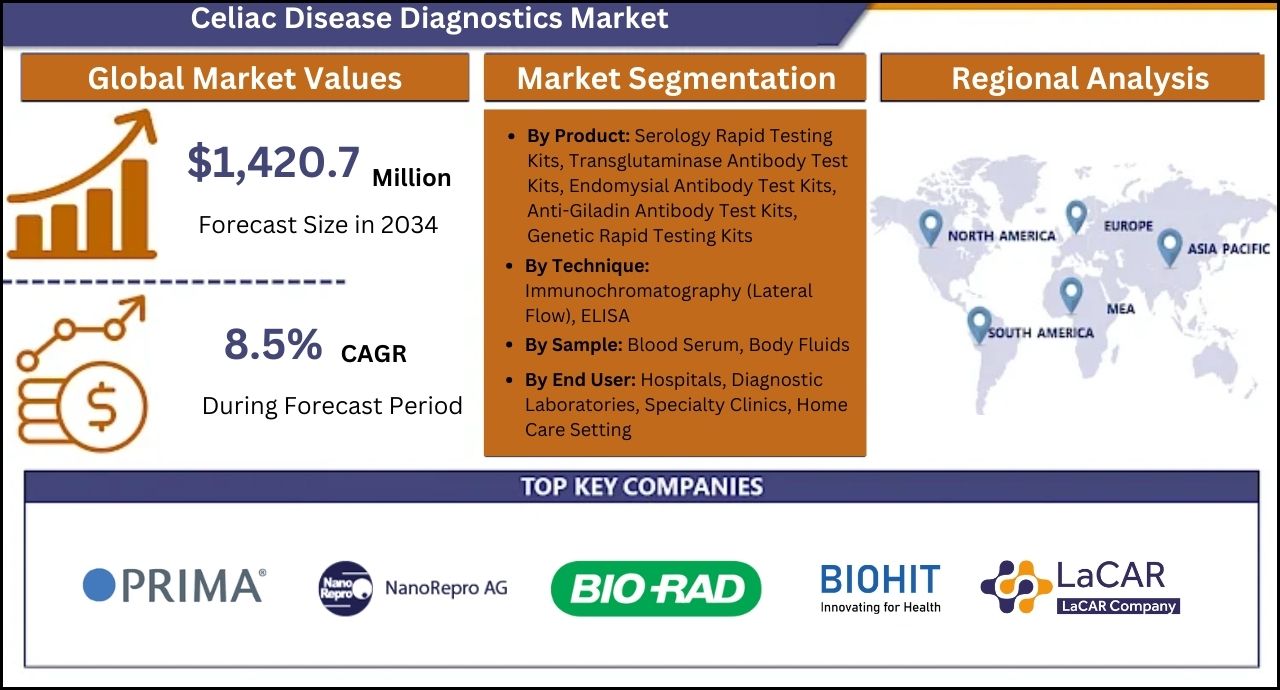
- The global Celiac disease diagnostics market is projected to grow at 8.5% CAGR and reach US$ 1,420.7 million by 2034
- The market created an absolute $ opportunity of US$ 794.0 million growing at a CAGR of 8.5% between 2024 to 2034
- North America is a prominent region that is estimated to hold a market share of 42.8% in 2034
- Serology rapid testing kits are estimated to grow at a CAGR of 9.0% creating an absolute $ opportunity of US$ 619.0 million between 2024 and 2034.
- North America and Western Europe are expected to create an absolute $ opportunity of US$ 455.0 million collectively.
“Increasing Usage of Home-Test Kits Boosts the Market Growth” says a Fact.MR analyst.
Regional Analysis:
The United States Celiac Disease Diagnostics Market is valued at an estimated US$ 234.3 million in 2024 and is expected to grow at a CAGR of 8.4% through 2034. With the efforts of organizations like the Celiac Disease Foundation and the National Celiac Association, along with various medical institutions, public awareness about celiac disease has grown significantly. Increased education and media coverage have led to a rise in testing and diagnosis rates, as more people seek medical advice when experiencing symptoms of gluten intolerance. This, combined with enhanced knowledge among healthcare providers, especially pediatricians, has fueled the market's growth trajectory.
Leading Players Driving Innovation in Celiac Disease Diagnostics Market:
Thermo Fisher Scientific Inc.; PRIMA Lab SA; Glutenostics, Inc.; NanoRepro AG; Targeted Genomics; Bio-Rad Laboratories Inc.; Biohit Oyj; Labsystems Diagnostics Oy; RxHome Test; AESKU.GROUP GmbH; LaCAR MDx Technologies; Vitrosens Biotechnology; Inova Diagnostics; EmpowerDx (Eurofins Scientific); Everlywell, Inc.
Market Development:
The home test kits are increasingly popular as the method of diagnosing celiac disease, thus boosting the market. Many equipment makers offer kits for measuring gluten responses and thereby show the likelihood of getting the disease.
Blood or swab are generally used in testing and these tests can be sent to a laboratory so as to analyse results collected during a home test. All results obtained in labs are usually easy to comprehend, and which can be accessible in 5-7 working days.
Some examples of home-test kits include imaware Celiac Disease Screening Test, Targeted Genomics Gluten ID Test, LetsGetChecked Celiac Test, EmpowerDX Celiac Risk Gene Test and Genovate DNA Celiac Disease Test. There is increasing involvement of different manufacturers in more product releases of at-home test kits. For example, empowerDX launched a simple test for Celiac Disease Genetic Risk on May 5, 2022. Hence, it is anticipated that the rise in usage of home-test kits will promote market expansion during the prediction time.
Celiac Disease Diagnostics Industrial News:
- Inova Diagnostics acquired FDA (510k) clearance in September 2021 for their Aptiva System and IgA test for coeliac disease. Because of its fully automated digital system, clinical laboratories may now handle data at high throughput in the next generation.
- New nutritional and digestive health solutions, including at-home coeliac disease collecting tests, were announced by Everlywell, Inc. in June 2022.
- EmpowerDx and NIMA inked a collaboration agreement in September 2022 to jointly market EmpowrDx's genetic test for coeliac disease identification.
- To develop ZED1227/TAK-227, a Phase 2b experimental therapeutic for coeliac disease, Takeda and Zedira and Dr. Falk Pharma agreed into a partnership and licensing agreement in October 2022.
Get Customization on this Report for Specific Research Solutions: https://www.factmr.com/connectus/sample?flag=S&rep_id=10338
More Valuable Insights on Offer:
Fact.MR, in its new offering, presents an unbiased analysis of the global celiac disease diagnostics market, presenting historical data for 2019 to 2023 and forecast statistics for 2024 to 2034.
The study reveals essential insights on the basis of the Product (Serology Rapid Testing Kits, Genetic Rapid Testing Kits), Technique (Immunochromatography and ELISA), Sample (Blood Serum and Body Fluids), and End User (Hospitals, Diagnostic Laboratories, Specialty Clinics, and Home Care Setting) across major regions of the world (North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, and Middle East & Africa).
Check out More Related Studies Published by Fact.MR Research:
Blood Cancer Diagnostics Market: In FY 2021, the blood cancer diagnostics market reached a valuation of US$ 15.05 Billion, and is likely to register a Y-o-Y growth rate of 5.6% in 2022, closing at US$ 15.95 Billion.
Ovarian Cancer Diagnostics Market: This global ovarian cancer diagnostics market analysis by Fact.MR predicts the industry to expand at a robust CAGR of 7% from 2021 to 2031.
Breast Cancer Diagnostics Market: The global breast cancer diagnostics market is likely to acquire a market value of US$ 4 Bn in 2022 and is expected to register a CAGR of 7% by accumulating a market value of US$ 7.9 Bn in the forecast period 2022-2032.
Kidney Cancer Diagnostics Market: The global kidney cancer diagnostics market is projected to expand steadily at a CAGR 7% value, during the forecast period 2022-2032. In the year 2022, the market size is projected to expand and gain a global market valuation of US$ 800 Million
About Us:
Fact.MR is a distinguished market research company renowned for its comprehensive market reports and invaluable business insights. As a prominent player in business intelligence, we deliver deep analysis, uncovering market trends, growth paths, and competitive landscapes. Renowned for its commitment to accuracy and reliability, we empower businesses with crucial data and strategic recommendations, facilitating informed decision-making and enhancing market positioning.
With its unwavering dedication to providing reliable market intelligence, FACT.MR continues to assist companies in navigating dynamic market challenges with confidence and achieving long-term success. With a global presence and a team of experienced analysts, FACT.MR ensures its clients receive actionable insights to capitalize on emerging opportunities and stay competitive.
Follow Us: LinkedIn | Twitter | Blog
