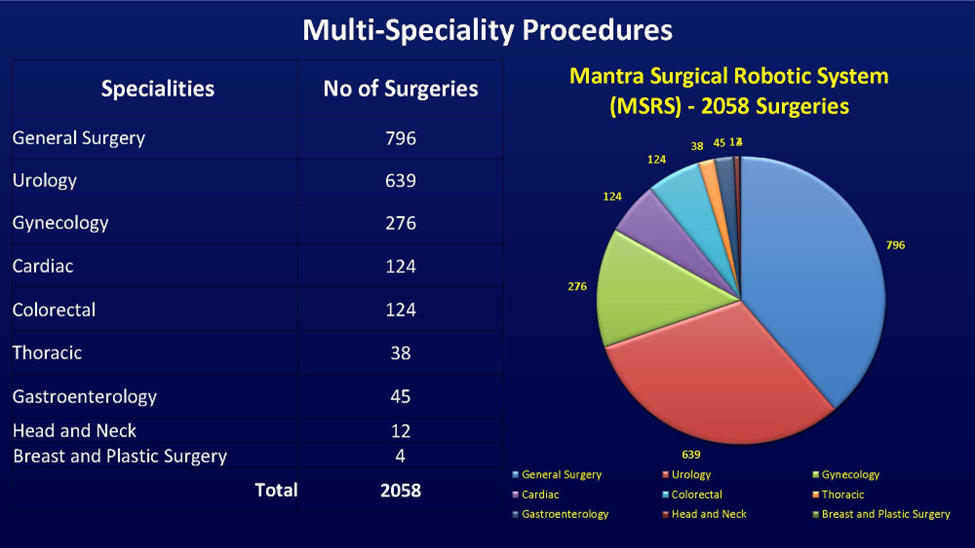
FORT LAUDERDALE, Fla., Sept. 23, 2024 (GLOBE NEWSWIRE) -- SS Innovations International, Inc. (the “Company” or “SS Innovations”) (OTC: SSII), developer of innovative surgical robotic technologies dedicated to making world-class robotic surgery affordable and accessible to a global population, announced that it has now successfully completed over 2,000 robotic surgeries, primarily in India, using its SSi Mantra Surgical Robotic System (the “SSi Mantra”).
The significance of the 2,000 surgeries extends beyond mere numbers; it embodies a critical response to a pressing need in the medical community. As global disparities in healthcare persist, SS Innovations has emerged as a leader in providing advanced surgical solutions that are accessible to all. The Company’s guiding principles of “democratizing access” and “decentralizing excellence” reflect its dedication to making world-class healthcare attainable for every patient, irrespective of their socio-economic background.
The SSi Mantra’s impact is already being felt in hospitals throughout India where it has been installed. Surgeons have praised its ergonomic design and precise controls, which facilitate minimally invasive procedures and improve patient outcomes. By minimizing recovery time and reducing the risk of complications, the SSi Mantra has become an invaluable tool in surgical suites, particularly in resource-constrained environments where access to advanced technologies has historically been limited due to the relative exorbitant cost associated with such technology.
Notably, the SSi Mantra has also been installed in many Tier 2 and Tier 3 cities across India, including Moradabad, Hisar, Coimbatore, Rohtak, and Cuttack, where communities are now reaping the benefits of innovative surgical technologies. Furthermore, the SSi Mantra also marks a significant milestone as Nepal’s first surgical robot, bringing renewed hope for advanced medical care to that nation.
Among the 2,000 surgeries performed, 123 were cardiac cases. Each procedure addresses varying complexities, highlighting the system’s versatility and adaptability in handling intricate surgical procedures.
To celebrate this milestone, SS Innovations Founder, Chairman, and CEO, Dr. Sudhir Srivastava expressed his gratitude to the entire team. “It is the spirit of our engineers, their faith in the system, and their commitment to improving lives that has brought us this far”.
Dr. Vishwa Srivastava, President and COO of SS Innovations, remarked, “Our mission as a digital healthcare company is to democratize global access to the latest technologies. This milestone underscores the impact of our innovations and drives us forward in our journey to improve surgical outcomes globally.”
The success of the SSi Mantra Surgical Robotic System in India has resulted in robust quality and safety data as well as clinical patient-centric outcomes. This should be helpful in not only continuing SS Innovation’s mission to provide quality care to patients across the globe but also of value in seeking further regulatory approval in other markets.
About SS Innovations International, Inc.:
SS Innovations International, Inc. (OTC: SSII) is a developer of innovative surgical robotic technologies with a vision to make the benefits of robotic surgery affordable and accessible to a larger part of the global population. SSII’s product range includes its proprietary “SSi Mantra” surgical robotic system, and “SSi Mudra” its wide range of surgical instruments capable of supporting a variety of surgical procedures including robotic cardiac surgery. SSII’s business operations are headquartered in India and SSII has plans to expand the presence of its technologically advanced, user-friendly, and cost-effective surgical robotic solutions, globally. For more information, visit SSII’s website at ssinnovations.com or LinkedIn for updates.
About SSI Mantra:
Supporting advanced, affordable, and accessible robotic surgery, the SSi Mantra Surgical Robotic System provides the capabilities for multi-specialty usage including cardiothoracic, head and neck, gynecology, urology, general surgery and more. With its modular arm configuration, 3D 4K vision open-console design and superior ergonomics, the system engages with the surgeon and surgical teams to improve safety and efficiency during procedures. The SSi Mantra system has received Indian Medical Device regulatory approval (CDSCO) and is clinically validated in India in more than 80 different types of surgical procedures. SS Innovations has commenced the regulatory approval process in the United States and the European Union and anticipates receiving FDA approval to market and CE Mark approval in the second half of 2025.
Forward-Looking Statements:
This press release may contain statements that are not historical facts and are considered forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. The words “anticipate,” “assume,” “believe,” “estimate,” “expect,” “will,” “intend,” “may,” “plan,” “project,” “should,” “could,” “seek,” “designed,” “potential,” “forecast,” “target,” “objective,” “goal,” or the negatives of such terms or other similar expressions to identify such forward-looking statements. These statements relate to future events or SS Innovations International, Inc.’s future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Follow us on:
LinkedIn: https://www.linkedin.com/company/ssinnovationsgroup/
YouTube: https://www.youtube.com/@ssinnovations
For media inquiries, please contact:
press@ssinnovations.org
+1-212-739-0300
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/60c19822-1f18-4438-a100-2f7290ba85d4
