New York, USA, Oct. 09, 2024 (GLOBE NEWSWIRE) -- Schizophrenia Clinical Trial Pipeline Insights Featuring 55+ Companies | DelveInsight
The rise in research and development activities in schizophrenia treatment is driving market growth by fostering the development of innovative therapies, including novel antipsychotics and personalized medicine. Advancements in neurobiology and biomarkers are enhancing drug discovery, while clinical trials for more effective, targeted treatments continue to expand. This increased focus on innovation is attracting significant investments, boosting the schizophrenia market.
DelveInsight’s 'Schizophrenia Pipeline Insight 2024' report provides comprehensive global coverage of pipeline schizophrenia in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the schizophrenia pipeline domain.
Key Takeaways from the Schizophrenia Pipeline Report
- DelveInsight’s schizophrenia pipeline report depicts a robust space with 55+ active players working to develop 60+ pipeline schizophrenia drugs.
- Key schizophrenia companies such as Sumitomo Pharma America, Boehringer Ingelheim, Merck Sharp & Dohme, MapLight Therapeutics, Reviva Pharmaceuticals, Newron Pharmaceuticals, Vanda Pharmaceuticals, Celon Pharma, Zhejiang Jingxin Pharmaceutical, Jiangsu Hansoh Pharmaceutical, Kynexis, Cerevance, Terran Biosciences, Luye Pharma Group, Gabather, Oryzon Genomics, Fabre-Kramer Pharmaceuticals, Zhejiang Jingxin Pharmaceutical, NeuShen Therapeutic, Stalicla, and others are evaluating new schizophrenia drugs to improve the treatment landscape.
- Promising pipeline schizophrenia such as Ulotaront, Iclepertin, MK-8189, ML-007, VHX 896, Brilaroxazine, Evenamide, CPL 500036, JX 11502, MAHS-10509, KYN-5356, CVN766, TerXT, LY03020, GT-002, Vafidemstat, FKF02SC, JX11502MA, NS-136, STP2, and others are under different phases of schizophrenia clinical trials.
- In September 2024, Reviva Pharmaceuticals presented new vocal biomarker data from the Phase III RECOVER trial of brilaroxazine in schizophrenia during a virtual key opinion leader event hosted by the company.
- In August 2024, Luye Pharma Group announced that the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) had approved its Investigational New Drug (IND) application for LY03020 filed under the Class 1 pathway for investigational drugs. LY03020, a dual TAAR1/5-HT2CR agonist, is intended to treat schizophrenia and Alzheimer's disease psychosis (ADP).
- In May 2024, Teva announced positive results from the Phase III SOLARIS trial, evaluating TEV-749 in adult patients with schizophrenia.
- In May 2024, Terran Biosciences Inc. revealed the creation of TerXT, a combination therapy featuring new prodrugs of xanomeline and trospium, aimed at providing long-lasting treatment for schizophrenia.
- In May 2024, NeuShen Therapeutics announced the dosing of the first healthy volunteer in Australia in the Phase I first-in-human clinical trial of NS-136 for the treatment of schizophrenia and other conditions associated with psychosis.
- In April 2024, Gabather AB announced that the Company has signed a Collaborative Agreement with the Centre for Neuropsychiatric Schizophrenia Research at the Psychiatric Centre in Glostrup to conduct a so-called clinical phase II study with GT-002 in patients diagnosed with schizophrenia.
- In March 2024, Sumitomo Pharma Co., Ltd. and its US-based subsidiary Sumitomo Pharma America, Inc. announced that they have agreed to amend the collaboration and license agreement for the worldwide joint development and commercialization of the four investigational candidate compounds, including ulotaront, under development in the psychiatry and neurology area, initially concluded between Sumitomo Pharma, SMPA, and Otsuka Pharmaceutical Co., Ltd. on September 30, 2021.
- In March 2024, Boehringer Ingelheim and Sosei Group Corporation announced that they had entered a global collaboration and exclusive option-to-license agreement. At the center is a joint mission to develop and commercialize Sosei Heptares’ portfolio of first-in-class GPR52 agonists, a novel G protein-coupled receptor (GPCR) target, with the intent to improve patient outcomes by simultaneously addressing positive, negative, and cognitive symptoms of schizophrenia.
- In February 2024, the FDA denied approval for an experimental schizophrenia drug developed by Minerva Neurosciences, dismissing the biotechnology company's efforts to gain authorization despite the agency's concerns.
Request a sample and discover the recent advances in schizophrenia drugs @ Schizophrenia Pipeline Report
The schizophrenia pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage schizophrenia drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the schizophrenia clinical trial landscape.
Schizophrenia Overview
Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels, and behaves. People with schizophrenia often seem disconnected from reality, which can be distressing for both the individual and their loved ones. The exact cause of schizophrenia is unknown, but it is believed to result from a complex interplay of genetic, environmental, and neurochemical factors. Brain structure abnormalities and imbalances in neurotransmitters, such as dopamine, are also implicated.
Symptoms of schizophrenia typically include hallucinations, delusions, disorganized thinking, and speech, as well as abnormal motor behavior. Negative symptoms, such as diminished emotional expression and lack of motivation, are also common.
Schizophrenia diagnosis involves a comprehensive evaluation by a psychiatrist, including clinical interviews, observation, and the exclusion of other mental health disorders or medical conditions that could cause similar symptoms. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) provides criteria for diagnosis, including persistent symptoms for at least six months.
Schizophrenia treatment usually requires a combination of antipsychotic medications, which help reduce the intensity of hallucinations and delusions, along with psychotherapy and social support. Cognitive Behavioral Therapy (CBT) and family therapy are often helpful in managing the emotional and social challenges of the disorder. In severe cases, hospitalization may be required to ensure the safety of the patient and others. Although schizophrenia is a lifelong condition, early and consistent treatment can help manage symptoms and improve quality of life.
First-generation antipsychotics such as chlorpromazine, fluphenazine, haloperidol, and perphenazine are effective in managing positive symptoms like hallucinations and delusions, but they do not improve negative symptoms or cognitive deficits.
Second-generation antipsychotics, including REXULTI/RXULTI (brexpiprazole), CAPLYTA (lumateperone), LATUDA (lurasidone hydrochloride), SAPHRIS (asenapine), ABILIFY MYCITE (aripiprazole with a sensor), VRAYLAR/REAGILA (cariprazine), SECUADO (asenapine), INVEGA SUSTENNA/TRINZA/HAYFERA (paliperidone palmitate), ARISTADA/ARISTADA INITIO (aripiprazole lauroxil), PERSERIS (risperidone), FANAPT (iloperidone), and LYBALVI (olanzapine and samidorphan), were developed to target both positive and negative symptoms, along with cognitive impairments.
While these drugs tend to have fewer extrapyramidal side effects, they are associated with risks such as weight gain, sexual dysfunction, and metabolic issues. Additionally, elderly patients using either FGAs or SGAs face a heightened risk of pneumonia.
Recently, BMS's innovative drug, KarXT—now branded as COBENFY—gained notable FDA approval on September 26, 2024, for treating adults with schizophrenia. COBENFY is the first muscarinic agonist to be approved for this condition, representing the first new class of treatment since CLOZARIL (clozapine) was approved by the FDA 35 years ago.
Find out more about schizophrenia drugs @ Schizophrenia Analysis
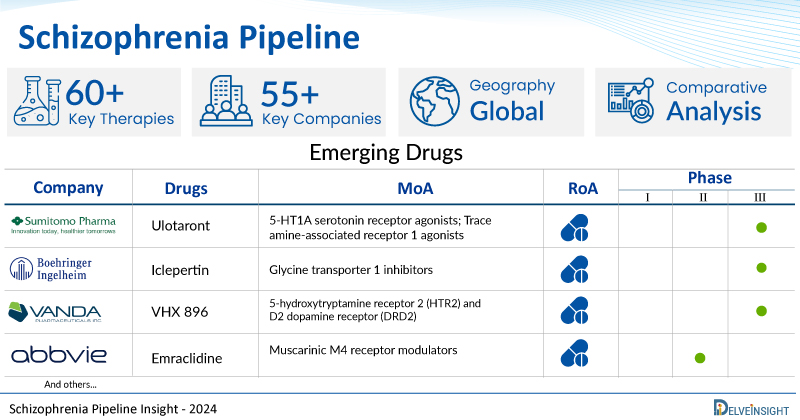
A snapshot of the Pipeline Schizophrenia Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Ulotaront | Sumitomo Pharma America | III | 5-HT1A serotonin receptor agonists; Trace amine-associated receptor 1 agonists | Oral |
| Iclepertin | Boehringer Ingelheim | III | Glycine transporter 1 inhibitors | Oral |
| Brilaroxazine | Reviva Pharmaceuticals | III | Dopamine D2 receptor partial agonists; Dopamine D3 receptor partial agonists; Dopamine D4 receptor partial agonists; Serotonin 1A receptor partial agonists; Serotonin 2A receptor partial agonists; Serotonin 6 receptor antagonists; Serotonin 7 receptor antagonists | Oral |
| VHX 896 | Vanda Pharmaceuticals | III | 5-hydroxytryptamine receptor 2 (HTR2) and D2 dopamine receptor (DRD2) | Oral |
| Emraclidine | AbbVie | II | Muscarinic M4 receptor modulators | Oral |
| ALTO-101 | Alto Neuroscience | II | Type 4 cyclic nucleotide phosphodiesterase inhibitors | Transdermal |
| NBI-1117568 | Neurocrine Biosciences/ Nxera Pharma | II | Muscarinic M4 receptor agonists | Oral |
| CPL 500036 | Celon Pharma | II | Phosphodiesterase 10A inhibitors | Oral |
| JX 11502MA | Zhejiang Jingxin Pharmaceutical | I/II | Dopamine D2 receptor agonists; Dopamine D3 receptor agonists | Oral |
Learn more about the emerging Schizophrenia therapies @ Schizophrenia Clinical Trials
Schizophrenia Therapeutics Assessment
The schizophrenia pipeline report proffers an integral view of the emerging schizophrenia segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Schizophrenia Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: 5-HT1A serotonin receptor agonists, Trace amine-associated receptor 1 agonists, Dopamine D4 receptor partial agonists, Serotonin 1A receptor partial agonists, Serotonin 2A receptor partial agonists, Serotonin 6 receptor antagonists, Serotonin 7 receptor antagonists, Type 4 cyclic nucleotide phosphodiesterase inhibitors, Muscarinic M4 receptor agonists, Phosphodiesterase 10A inhibitors, Dopamine D2 receptor agonists, Dopamine D3 receptor agonists,
- Key Schizophrenia Companies: Sumitomo Pharma America, Boehringer Ingelheim, Merck Sharp & Dohme, MapLight Therapeutics, Reviva Pharmaceuticals, Newron Pharmaceuticals, Vanda Pharmaceuticals, Celon Pharma, Zhejiang Jingxin Pharmaceutical, Jiangsu Hansoh Pharmaceutical, Kynexis, Cerevance, Terran Biosciences, Luye Pharma Group, Gabather, Oryzon Genomics, Fabre-Kramer Pharmaceuticals, Zhejiang Jingxin Pharmaceutical, NeuShen Therapeutic, Stalicla, and others
- Key Schizophrenia Pipeline Therapies: Ulotaront, Iclepertin, MK-8189, ML-007, VHX 896, Brilaroxazine, Evenamide, CPL 500036, JX 11502, MAHS-10509, KYN-5356, CVN766, TerXT, LY03020, GT-002, Vafidemstat, FKF02SC, JX11502MA, NS-136, STP2, and others
Dive deep into rich insights for new schizophrenia treatments, visit @ Schizophrenia Drugs
Table of Contents
| 1. | Schizophrenia Pipeline Report Introduction |
| 2. | Schizophrenia Pipeline Report Executive Summary |
| 3. | Schizophrenia Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Schizophrenia Clinical Trial Therapeutics |
| 6. | Schizophrenia Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Schizophrenia Pipeline: Late-Stage Products (Phase III) |
| 8. | Schizophrenia Pipeline: Mid-Stage Products (Phase II) |
| 9. | Schizophrenia Pipeline: Early-Stage Products (Phase I) |
| 10. | Schizophrenia Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Schizophrenia Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Schizophrenia Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the schizophrenia pipeline therapeutics, reach out @ Schizophrenia Therapeutics
Related Reports
Schizophrenia Epidemiology Forecast
Schizophrenia Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and schizophrenia epidemiology trends.
Schizophrenia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key schizophrenia companies, including Boehringer Ingelheim, Sumitomo Pharma, Otsuka, Reviva Pharmaceutical, Minerva Neurosciences, Mitsubishi Tanabe Pharma, Karuna Therapeutics (Bristol Myers Squibb), Royalty Pharma, ACADIA Pharmaceuticals, Teva Pharmaceutical, MedinCell, Royalty Pharma, Neurocrine Biosciences, Lyndra Therapeutics, Newron Pharmaceuticals, Luye Pharma, among others.
Cognitive Impairment Associated with Schizophrenia Market
Cognitive Impairment Associated with Schizophrenia Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cognitive impairment associated with schizophrenia companies, including Boehringer Ingelheim, Neurocrine Biosciences, Recognify Life Sciences, Atai Life Sciences, Kynexis, among others.
Dementia Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key dementia companies, including Hoffmann-La Roche, Forest Laboratories, Inc., Janssen Pharmaceuticals, Inc., Merck and Co., Inc., Novartis, among others.
Bipolar Depression Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bipolar depression companies, including Intra-Cellular Therapies, Sunovion Pharmaceuticals, COMPASS Pathways, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
