-Transenteric delivery of incretin triagonist GLP-1, GIP, glucagon receptors mimicking the RaniPill route of administration elicits rapid weight loss and bioavailability comparable to subcutaneous injection -
- New pharmacokinetic data provides further evidence of the RaniPill platform’s potential to enable oral delivery of multiple obesity treatments -
- Phase 1 study for RT-114, an oral GLP-1/GLP-2 dual agonist for the treatment of obesity, expected to initiate in 2025 -
SAN JOSE, Calif., Oct. 17, 2024 (GLOBE NEWSWIRE) -- Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today announced new pharmacokinetic data from a preclinical study evaluating a GLP-1, GIP and glucagon receptors incretin triagonist with a delivery method mimicking the RaniPill route of administration. A previous study with this incretin triagonist delivered transenterically demonstrated pharmacodynamic effects comparable to subcutaneous injection. Rani also previously completed a study demonstrating oral delivery of GLP-1 receptor agonist with high bioavailability via the RaniPill capsule.
“The pharmacokinetic data announced today combined with the previously announced pharmacodynamic data further highlight the RaniPill’s potential to serve as a novel delivery platform for incretin triagonists, a next generation modality for the treatment of obesity,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “Overall, the totality of RaniPill preclinical data presented to date is promising and provides scientific validation for the potential of the RaniPill to replace painful injections with oral alternatives with differentiated dosing flexibility for multiple obesity drug candidates. Looking ahead, we are focused on execution of a Phase 1 clinical trial for RT-114, a RaniPill capsule containing ProGen’s GLP-1 / GLP-2 dual-agonist, PG-102, and we are evaluating options to move forward with one or more additional molecules in the obesity space.”
Data Highlights
The preclinical study evaluated the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of an incretin triagonist (GLP-1, GIP, glucagon receptors) when delivered via an endoscope-guided transenteric administration to mimic the RaniPill route of administration, versus the traditional administration route of subcutaneous (SC) injection. The study was conducted in canines separated into two groups. In Group 1 (N=3), 0.12 mg/kg of drug was administered via transenteric delivery by endoscope. In Group 2 (N=5), 0.12 mg/kg of drug was administered by SC injection. Blood samples were collected over 2 weeks for analysis of serum drug concentrations and various PD and safety biomarkers.
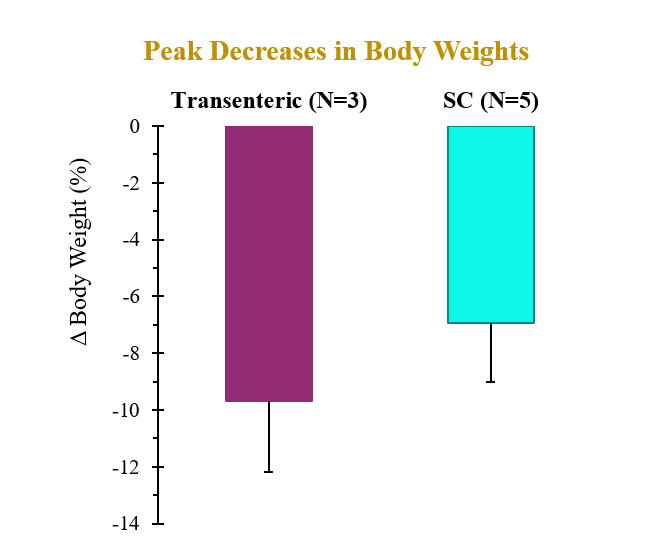
A single dose of drug delivered via either transenteric or SC routes elicited rapid decreases in body weight and serum lipids. Weight loss observed following transenteric delivery was 9.7 ± 2.5 % versus 6.9 ± 2.1 % following SC injection and is believed to be due to early satiety leading to reduced caloric intake. Additionally, the bioavailability of drug delivered via the transenteric route was comparable to that of drug delivered via the SC route at the same dose. The drug was well tolerated in both groups with no serious adverse events (SAEs) observed or changes in safety markers examined.
Transenteric delivery yielded 80% relative bioavailability versus SC and differences between PK parameters (AUC, Cmax, Tmax) were not statistically significant.
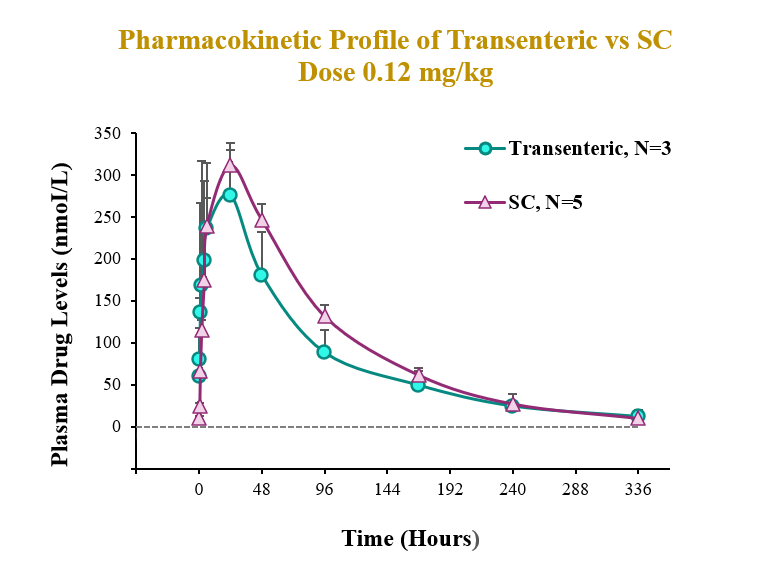
| AUC (nmol*h/L) | Cmax (nmol/L) | Tmax (h) | |
| Transenteric | 26963.3 ± 5520.0 | 334.3 ± 32.8 | 16.7 ± 10.4 |
| SC | 33509.5 ± 1576.1 | 311.5 ± 26.1 | 24 ± 0.0 |
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill® capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill® capsule technology. For more information, visit ranitherapeutics.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the potential opportunity for the RaniPill platform to enable oral delivery of multiple obesity drug products, the potential for the transenteric delivery via endoscope of an incretin triagonist to mimic the RaniPill route of delivery, the expected initiation of a Phase 1 trial of RT-114 in 2025, the potential for the RaniPill to serve as a novel delivery platform for incretin triagonists, the likelihood that weight loss in the preclinical study of the incretin triagonist was caused by early satiety leading to reduced caloric intake, the belief that the preclinical data are reflective of the potential contributions the RaniPill capsule can make to the GLP-1 receptor agonist space and the broader obesity market, and the potential for Rani to use the RaniPill platform to replace painful injections with oral alternatives with differentiated dosing flexibility for multiple obesity drug candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believed,” “potential,” “expected,” “looking ahead,” “intended” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2023, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Trademarks
Trade names, trademarks and service marks of other companies appearing in this press release are the property of their respective owners. Solely for convenience, the trademarks and trade names referred to in this press release appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
Investor Contact:
investors@ranitherapeutics.com
Media Contact:
media@ranitherapeutics.com
Charts accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/cd69bfb8-05d6-4457-b231-74fbae7b6537
https://www.globenewswire.com/NewsRoom/AttachmentNg/4dc9ac35-87a7-48d7-a985-335cbeb40650
