New York, USA, Oct. 22, 2024 (GLOBE NEWSWIRE) -- Gene Therapy Clinical Trial Pipeline Gains Momentum: 180+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
The gene therapies market is experiencing significant growth, driven by advancements in research and technology. According to DelveInsight, the gene therapy clinical trial pipeline features over 180 key companies actively engaged in the development of more than 200 gene therapies. This dynamic landscape highlights the increasing investment and innovation in the sector, promising new treatment options for various diseases.
DelveInsight’s 'Gene Therapy Competitive Landscape – 2024' report provides comprehensive global coverage of available, marketed, and pipeline gene therapies in various stages of clinical development, major pharmaceutical companies working to advance the pipeline space and future growth potential of the gene therapy competitive domain.
Key Takeaways from the Gene Therapy Pipeline Report
- Over 180+ companies are evaluating 200+ gene therapies in various stages of development, and their anticipated acceptance in the gene therapy market would significantly increase market revenue.
- Leading gene therapy companies such as Ultragenyx Pharmaceutical Inc, Rocket Pharmaceuticals, Adverum Biotechnologies, Kyverna Therapeutics, Vivet Therapeutics, HELIXMITH Co, Rocket Pharmaceuticals, Bristol-Myers Squibb, Wellington Zhaotai Therapies, Reyon Pharmaceutical, Neowise Biotechnology, Orna Therapeutics, Genflow Biosciences, Xylocor Therapeutics, Kolon TissueGene, Allogene Therapeutics, Nanjing IASO Biotherapeutics, Shanghai Vitalgen BioPharma, Rui Therapeutics, Obsidian Therapeutics, YolTech Therapeutics, Neurotech USA, Beacon Therapeutics, REGENXBIO, and others are evaluating novel gene therapy candidates to improve the treatment landscape.
- Key gene therapies in the pipeline in various stages of development include DTX401, RP-L102, ADVM-022, KYV-101, VTX-802, RP A501, CD19-NEX-T, GP28 zT2, RY 105, WZTL 002, NW 301V, Research programme: in-vivo CRISPR editing gene therapies, Research programme: sarcopenia therapeutics, Encoberminogene rezmadenovec, Tonogenchoncel-L, ALLO 605, Equecabtagene autoleucel, VGN R09, KN 5501, OBX 115, YOLT 201, Revakinagene taroretcel, Laruparetigene zosaparvovec, RGX 121, and others.
Request a sample and discover the recent advances in the gene therapy market @ Gene Therapy Competitive Landscape Report
Gene Therapy Overview
Gene therapy is a groundbreaking medical approach that aims to treat or prevent diseases by directly modifying the genes within an individual's cells. By delivering therapeutic genes into a patient’s cells, this technique seeks to address the underlying genetic defects that contribute to various diseases, particularly genetic disorders, cancers, and certain viral infections. The fundamental principle of gene therapy involves replacing faulty genes, inactivating or knocking out disease-causing genes, or introducing new genes to help fight a disease. This innovative treatment holds the potential to transform the management of previously incurable conditions, offering hope to patients with rare genetic disorders or advanced diseases.
One of the most promising aspects of gene therapy is its ability to provide long-lasting effects with potentially fewer side effects compared to traditional treatments like chemotherapy or long-term use of medications. Advances in technologies such as CRISPR/Cas9 and viral vectors have significantly improved the precision and efficiency of gene delivery systems. These methods enable researchers and clinicians to target specific genes with greater accuracy, reducing the risk of unintended genetic changes. Clinical trials have shown encouraging results, with some gene therapies successfully curing conditions such as spinal muscular atrophy and certain types of inherited blindness.
However, gene therapy is not without challenges and ethical considerations. Issues such as the potential for immune reactions to the viral vectors used for gene delivery, the long-term effects of gene modification, and concerns regarding germline editing raise important questions about the safety and regulation of these therapies. Additionally, the high cost of development and treatment presents barriers to accessibility for many patients. As research continues and the field evolves, it will be crucial to balance the promise of gene therapy with thorough ethical oversight and equitable access to ensure that its benefits can be realized by all patients in need.
Gene Therapy Pipeline Analysis: Drug Profile
ZOLGENSMA: Novartis
ZOLGENSMA (onasemnogene abeparvovec) is the sole gene therapy for spinal muscular atrophy and the only treatment specifically designed to target the genetic cause of the disease. It works by replacing the function of the missing or defective SMN gene, effectively halting disease progression through sustained expression of SMN protein after a single intravenous infusion. The FDA approved ZOLGENSMA for pediatric patients under the age of 2 who have spinal muscular atrophy due to bi-allelic mutations in the survival motor neuron 1 (SMN1) gene. Currently, ZOLGENSMA has received approval in over 40 countries, with more than 2,000 patients treated worldwide through clinical trials, managed access programs, and commercial use.
LUXTURNA: Spark Therapeutics
LUXTURNA is the first gene therapy approved by the FDA for a genetic condition, making it the only pharmacological treatment available for inherited retinal diseases. It is also the first AAV vector gene therapy approved in the US. The FDA granted LUXTURNA Priority Review status and it has previously been designated as an orphan drug and breakthrough therapy. Following this approval, the FDA will provide Spark Therapeutics with a Rare Pediatric Disease Priority Review Voucher to expedite the review of a future marketing application for another product. Currently, Spark Therapeutics' MAA for LUXTURNA is under review by the EMA, which has also granted it orphan product designations.
Find out more about FDA-approved gene therapies @ Gene Therapy Analysis
The gene therapy market is witnessing significant growth, driven by advancements in technology, increasing investments, and a growing understanding of genetic diseases. As researchers unlock the potential of gene editing tools like CRISPR, ZFNs, and TALENs, the landscape of gene therapy is evolving rapidly. These technologies enable precise modifications at the genetic level, paving the way for innovative treatments for a range of conditions, from rare genetic disorders to more common diseases like cancer. The rise in research activities and clinical trials highlights the expanding capabilities of gene therapy, leading to an increase in approved therapies and a promising pipeline of candidates.
Market dynamics are also influenced by regulatory frameworks and reimbursement policies. Authorities like the FDA and EMA are streamlining the approval process for gene therapies, recognizing their potential to address unmet medical needs. However, the complexity of these therapies presents challenges in terms of regulatory oversight and safety assessments. Additionally, the high costs associated with gene therapies have raised concerns about accessibility and reimbursement. Market players are collaborating with governments and insurance companies to establish pricing models that reflect the long-term value of these treatments, thus promoting wider adoption.
Furthermore, the competitive landscape is intensifying as established pharmaceutical companies and biotech startups alike seek to capitalize on the potential of gene therapy. Collaborations, mergers, and acquisitions are becoming increasingly common as companies aim to bolster their research and development capabilities and expand their product portfolios. Strategic partnerships with academic institutions and research organizations are also prevalent, as they provide access to cutting-edge research and innovations. This dynamic environment fosters innovation, enabling faster development and commercialization of new gene therapies.
Finally, public perception and awareness of gene therapy play a critical role in shaping market dynamics. As success stories emerge and media coverage increases, patient and healthcare provider awareness of the potential benefits of gene therapy grows. However, ethical considerations surrounding gene editing, especially in germline modifications, pose challenges that could impact public acceptance and regulatory policies. Educating stakeholders and fostering open dialogues about the benefits and risks of gene therapy will be essential for the market’s sustained growth and acceptance in the healthcare ecosystem.
To know more about gene therapies, visit @ Gene Therapy Market Insights
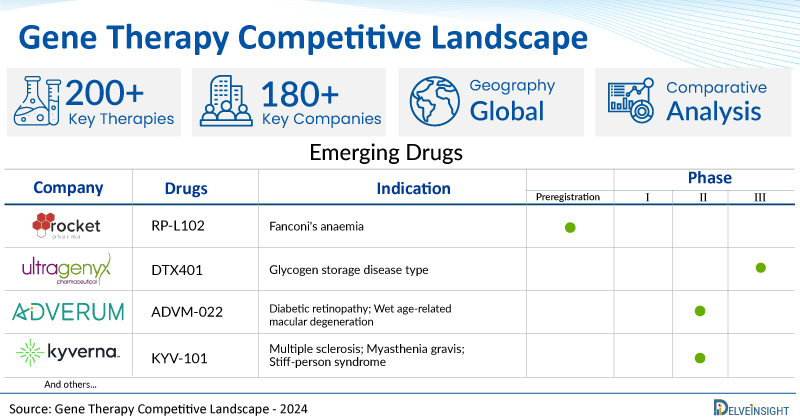
A snapshot of the pipeline gene therapies mentioned in the report:
| Gene Therapies | Company | Phase | Indication |
| RP-L102 | Rocket Pharmaceuticals | Preregistration | Fanconi's anaemia |
| DTX401 | Ultragenyx Pharmaceutical Inc | III | Glycogen storage disease type I |
| ADVM-022 | Adverum Biotechnologies | II | Diabetic retinopathy; Wet age-related macular degeneration |
| KYV-101 | Kyverna Therapeutics | II | Multiple sclerosis; Myasthenia gravis; Stiff-person syndrome |
| RP A501 | Rocket Pharmaceuticals | II | Glycogen storage disease type II |
| ALLO 605 | Allogene Therapeutics/Cellectis | I/II | Multiple myeloma |
| CD19-NEX-T | Juno Therapeutics | I | Multiple sclerosis; Non-Hodgkin's lymphoma; Systemic lupus erythematosus |
| NW 301V | Neowise Biotechnology | I | Solid tumors |
| WZTL 002 | Wellington Zhaotai Therapies | I | Non-Hodgkin's lymphoma |
| RY 105 | Reyon Pharmaceutical | Preclinical | Hepatic fibrosis |
Discover more about gene therapies in clinical development @ Gene Therapy in Clinical Trials
Key Developments in the Gene Therapy Domain
- In July 2024, Pfizer discontinued the phase III CIFFREO trial evaluating investigational gene therapy fordadistrogene movaparvovec. Earlier the gene therapy also did not achieve the study's main goal of enhancing motor function in boys aged 4 to 7 years compared to a placebo.
- In July 2024, Miltenyi Biotec announced the signing of a Letter of Intent with the Translational Health Science and Technology Institute (THSTI). With this partnership, both organizations aim to address the growing need for innovative treatments in the fight against cancer by developing innovative cell and gene therapies.
- In May 2024, Taysha Gene Therapies received a regenerative medicine advanced therapy (RMAT) designation for its gene therapy candidate TSHA-102 from the US Food and Drug Administration (FDA) to treat Rett syndrome.
- In April 2024, Charles River Laboratories International, Inc. announced a plasmid DNA contract development and manufacturing organization (CDMO) collaboration with Axovia Therapeutics Ltd. Charles River will manufacture High Quality (HQ) gene of interest plasmid to support the development of Axovia’s gene therapies for ciliopathies, including Bardet-Biedl Syndrome (BBS), a condition with limited treatment options and no cure.
- In April 2024, Ginkgo Bioworks successfully completed the gene therapy collaboration with Biogen announced in May 2021. The companies aimed to advance the industry standard for manufacturing recombinant adeno-associated virus (AAV)-based vectors. The collaboration achieved its goals of enhancing the AAV production titers of Biogen's gene therapy manufacturing processes.
- In March 2024, Seattle Children’s Research Institute and Genezen unveiled a strategic manufacturing partnership for Seattle Children’s Research Institute’s X-linked agammaglobulinemia (XLA) program. This collaboration is focused on leveraging Genezen’s viral vector process development and cGMP manufacturing expertise to advance Seattle Children’s XLA gene therapy program.
- In March 2024, Taysha Gene Therapies announced that they had received the needed approval to move forward with dose escalation in its Phase I/II trial testing TSHA-102, its gene therapy candidate for Rett syndrome, in adolescents and adults.
- In February 2024, the FDA approved the first cell therapy for a solid tumor, MIP Discovery secured £7 million in Series A financing to drive the commercialization of novel synthetic affinity reagents within the cell and gene therapy space, and Charles River Laboratories International has expanded its portfolio of human pluripotent stem cells through a collaboration with Pluristyx.
Scope of the Gene Therapy Competitive Landscape Report
- Coverage: Global
- Key Gene Therapy Companies: Ultragenyx Pharmaceutical Inc, Rocket Pharmaceuticals, Adverum Biotechnologies, Kyverna Therapeutics, Vivet Therapeutics, HELIXMITH Co, Rocket Pharmaceuticals, Bristol-Myers Squibb, Wellington Zhaotai Therapies, Reyon Pharmaceutical, Neowise Biotechnology, Orna Therapeutics, Genflow Biosciences, Xylocor Therapeutics, Kolon TissueGene, Allogene Therapeutics, Nanjing IASO Biotherapeutics, Shanghai Vitalgen BioPharma, Rui Therapeutics, Obsidian Therapeutics, YolTech Therapeutics, Neurotech USA, Beacon Therapeutics, REGENXBIO, and others
- Key Gene Therapies in Pipeline: DTX401, RP-L102, ADVM-022, KYV-101, VTX-802, RP A501, CD19-NEX-T, GP28 zT2, RY 105, WZTL 002, NW 301V, Research programme: in-vivo CRISPR editing gene therapies, Research programme: sarcopenia therapeutics, Encoberminogene rezmadenovec, Tonogenchoncel-L, ALLO 605, Equecabtagene autoleucel, VGN R09, KN 5501, OBX 115, YOLT 201, Revakinagene taroretcel, Laruparetigene zosaparvovec, RGX 121, and others.
Table of Contents
| 1. | Gene Therapy Pipeline Report Introduction |
| 2. | Gene Therapy Pipeline Report Executive Summary |
| 3. | Gene Therapy Pipeline: Overview |
| 4. | Gene Therapy Marketed Drugs |
| 4.1. | ZOLGENSMA: Novartis |
| 5. | Gene Therapy Clinical Trial Therapeutics |
| 6. | Gene Therapy Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Gene Therapy Pipeline: Late-Stage Products (Phase III) |
| 8. | Gene Therapy Pipeline: Mid-Stage Products (Phase II) |
| 8.1. | RP-L102: Rocket Pharmaceuticals |
| 9. | Gene Therapy Pipeline: Early-Stage Products (Phase I/II) |
| 9.1. | KYV-101: Kyverna Therapeutics |
| 10. | Gene Therapy Pipeline: Preclinical and Discovery Stage Products |
| 10.1. | VTX-802: Vivet Therapeutics |
| 11. | Gene Therapy Pipeline Therapeutics Assessment |
| 12. | Inactive Products in the Gene Therapy Pipeline |
| 13. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 14. | Unmet Needs |
| 15. | Gene Therapy Market Drivers and Barriers |
| 16. | Appendix |
For further information on gene therapy uses, reach out @ Viral Vectors for Gene Therapy
Related Reports
Gene Therapies In Ophthalmology Market
Gene Therapies In Ophthalmology Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gene therapies in ophthalmology companies, including BEACON THERAPEUTICS, NANOSCOPE THERAPEUTICS, COAVE THERAPEUTICS, BIONIC SIGHT, GENSIGHT BIOLOGICS, ADVERUM BIOTECHNOLOGIES, EYEVENSYS, EXEGENESIS BIO, MEIRAGTX, JOHNSON & JOHNSON INNOVATIVE MEDICINE, NEUROPHTH THERAPEUTICS, 4D MOLECULAR THERAPEUTICS, ATSENA THERAPEUTICS, OCUGEN, ABBVIE, REGENXBIO, SKYLINE THERAPEUTICS, HUIDAGENE THERAPEUTICS, OPUS GENETICS, among others.
Gene Therapies In Ophthalmology Competitive Landscape
Gene Therapies In Ophthalmology Competitive Landscape – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gene therapies in ophthalmology companies, including Spark Therapeutics, Regenxbio, Beacon Therapeutics, Adverum Biotechnologies, Exegenesis Bio, Frontera Therapeutics, HuidaGene Therapeutics, Nanjing IASO Biotherapeutics, GenSight Biologics, Sylentis, Neurophth Therapeutics, Johnson & Johnson Innovative Medicine, Nanoscope Therapeutics, Eyevensys, Atsena Therapeutics Inc., Coave Therapeutics, OCUGEN, INC, Visgenx, Amarna Therapeutics, Ikarovec, Homology Medicines, Ray Therapeutics, Shanghai Refreshgene Technology Co., Ltd., Complement Therapeutics, Abeona Therapeutics, among others.
Adeno-Associated Virus Vectors in Gene Therapy Market
Adeno-Associated Virus Vectors in Gene Therapy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key adeno-associated virus vectors in gene therapy companies, including Pfizer, CSL Behring, Spark Therapeutics, Freeline Therapeutics, RegenxBio, Amicus Therapeutics, among others.
Adeno-Associated Virus Vectors in Gene Therapy Pipeline
Adeno-Associated Virus Vectors in Gene Therapy Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key adeno-associated virus vectors in gene therapy companies, including Pfizer, CSL Behring, Spark Therapeutics, Freeline Therapeutics, RegenxBio, Amicus Therapeutics, among others.
Cell and Gene Therapies in Rare Disorders Market
Cell and Gene Therapies in Rare Disorders Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cell and gene therapies in rare disorders companies, including Roche, Freeline Therapeutics, Spark Therapeutics, Astellas Gene Therapies, Actus Therapeutics, GenSight Biologics, Coave Therapeutics, Johnson & Johnson, MeiraGTx, Applied Genetic Technologies Corporation, GenSight Biologics, Nanoscope Therapeutics, 4D Molecular Therapeutics, Ocugen, jCyte, ReNeuron, REGENXBIO, Amicus Therapeutics, Pfizer, Sarepta Therapeutics, Capricor Therapeutics, Nippon Shinyaku, Brainstorm Cell Therapeutics, CRISPR Therapeutics, Vertex Pharmaceuticals, Editas Medicine, Sangamo Therapeutics, Krystal Biotech, Abeona Therapeutics, Castle Creek Biosciences, Holostem Terapie Avanzate S.r.l., RHEACELL, Ishin Pharma, Anterogen, Ultragenyx Pharmaceutical, among others.
Rare Diseases Analysis: At DelveInsight, we are dedicated to providing essential reports that address the complexities of the rare cancer market. Our team of expert analysts diligently tracks the evolving developmental, regulatory, and commercial environments of competing products. We offer a range of services, including insightful conference evaluations and in-depth analyses of company progress and R&D activities, all designed to support the smooth development of oncology solutions tailored to our clients' specific requirements.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
