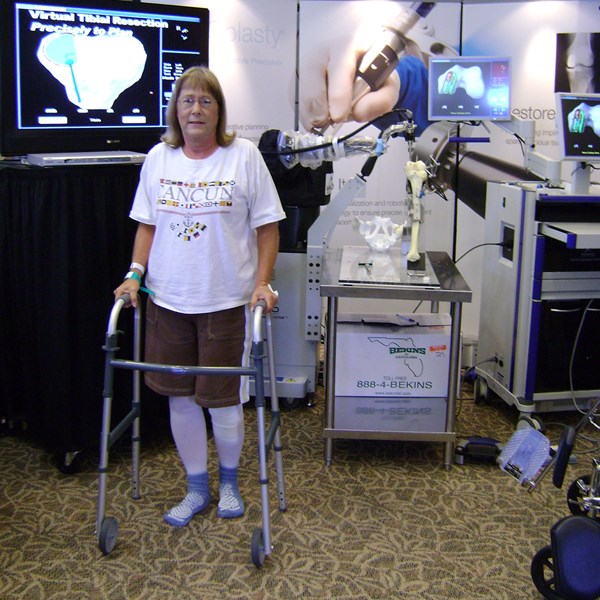
FORT LAUDERDALE, Fla., Sept. 30, 2008 (GLOBE NEWSWIRE) -- MAKO Surgical Corp. (Nasdaq:MAKO) hosted a live surgery during the second annual CIPKA meeting held on September 5 - 6, 2008 at the Mount Carmel New Albany Surgical Hospital Foundation Learning Center in New Albany, Ohio. CIPKA is a continuing medical education program designed to meet the educational needs of orthopedic surgeons, residents/fellows, nurses and physician assistants.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=5486
This year's two-day event focused on contemporary issues in partial knee arthroplasty (PKA) and afforded a faculty of worldwide experts an opportunity to review indications, contraindications and outcomes of medial, lateral, patellofemoral and bicompartmental replacements. The event was co-chaired by Keith R. Berend, MD, Joint Implant Surgeons, Inc.; Fred D. Cushner, MD, Director of Orthopaedics, Insall Scott Kelly Institute; and Adolph V. Lombardi, Jr., MD, FACS, President, Joint Implant Surgeons, Inc.
CIPKA's commitment to meeting the continuing medical educational needs of orthopedic surgeons was illustrated by a live surgical demonstration, during which more than 80 surgeons were able to view a surgeon-interactive robotic arm knee resurfacing procedure known as MAKOplasty(r). The procedure was performed by Jess H. Lonner, MD, Director of the Philadelphia Center for Minimally Invasive Knee Surgery, and broadcasted live to attendees in the state-of-the-art adjoining Learning Center.
"MAKO maintains a strong commitment to enhanced patient outcomes through the proliferation of clinical training, research and education," said Dr. Lonner. "There is great value to performing live surgery at educational programs. It gives attendees an unedited perspective into the nuances of a surgical procedure and technology," he added.
Surgeons in attendance also had the opportunity to gain hands-on experience with MAKO's proprietary Tactile Guidance System(tm) during a CIPKA sawbone lab session and learned how to apply robotic-arm technology to achieve precise bone resurfacing. MAKOplasty(r) facilitates accurate preparation of the femur and the tibia for unicompartmental knee resurfacing by combining robotic arm technology with patient-specific, three-dimensional virtual visualization -- affording surgeons new minimally invasive treatment options with reduced recovery time for patients with early to mid-stage osteoarthritis.
About MAKO Surgical Corp.
MAKO Surgical Corp. is a medical device company that markets its advanced robotic-arm solution and implants for minimally invasive orthopedic knee procedures. The MAKO Tactile Guidance System(tm) (TGS(tm)) is a surgeon-interactive tactile platform that incorporates a robotic arm and patient-specific visualization technology and prepares the knee joint for the insertion and alignment of resurfacing implants through a minimal incision. This FDA-cleared TGS allows surgeons to provide a tissue-sparing, bone resurfacing procedure called MAKOplasty(r) to a large, yet underserved patient-specific population suffering from early to mid-stage osteoarthritic knee disease. MAKO has an intellectual property portfolio of more than 200 licensed or owned patents and patent applications relating to the areas of computer assisted surgery, haptics, robotics and implants. Additional information can be found at www.makosurgical.com.
The photo is also available at Newscom, www.newscom.com, and via AP PhotoExpress.