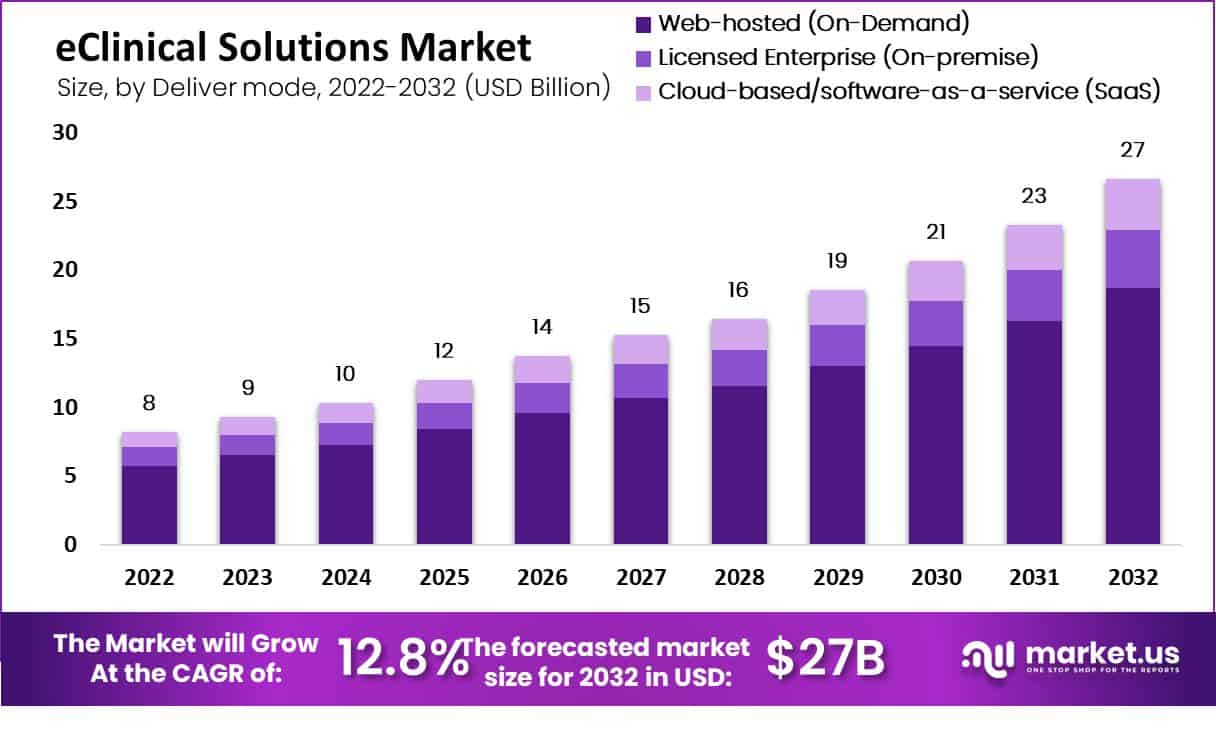
New York, April 25, 2023 (GLOBE NEWSWIRE) -- Market.us forecasts that the eClinical Solutions market will achieve a CAGR of 12.8% between 2023 and 2032, resulting in a market size of over USD 27 billion by 2032, up from USD 8 billion in 2022. Additionally, the development of the market for eClinical solutions in developed countries like the U.S. is greatly influenced by the existence of a strict regulatory framework for clinical trials and a greater requirement for safety monitoring. For instance, the National Institutes of Health and the U.S. Department of Health and Human Services are encouraging the exchange of clinical data and strengthening the criteria for registering clinical trials. One of the key factors driving the market for eClinical solutions is the soaring demand from pharmaceutical and biopharmaceutical businesses for software solutions for clinical trials. Moreover, expanding the end-user base for eClinical solutions and increasing government funds to support trials are predicted to drive the market for these products throughout the study period.
To get additional highlights on major revenue-generating segments, Request an eClinical Solutions Market sample report at https://market.us/report/eclinical-solutions-market/request-sample/
Key Takeaway:
- By Product, in 2022, Electronic Clinical Outcome Assessment (eCOA) dominate the eClinical market.
- By Mode Of Delivery, Due to the higher level of interoperability of these products, the web-hosted sector, with a market share of USD $2973.24 million, dominated the market in 2022.
- By Development Phase, the phase III sector of clinical trials dominated the eClinical solutions market in 2022, as more pharmaceuticals successfully advanced to phase III.
- By end-user, the Pharmaceutical & Biotechnology Companies dominate the market.
- In 2022, North America dominated the market with the highest revenue share.
Factors affecting the growth of eClinical Solutions?
- Increasing Adoption of Clinical Trials: As the pharmaceutical industry continues to grow, there is an increasing need for clinical trials to test new drugs and treatments. The adoption of eClinical solutions is growing as they offer an efficient and cost-effective way to manage these trials.
- Technological Advancements: The development of new technologies such as cloud computing, big data analytics, and mobile devices has enabled eClinical solutions to become more accessible, faster, and more efficient. This has led to increased adoption and growth of the eClinical solutions market.
- Need for Real-time Data: eClinical solutions provide real-time access to data from clinical trials, which can be crucial in decision-making. The ability to quickly and easily access data can lead to more efficient and effective clinical trial management, which is essential for drug development.
- Increasing Regulations: The pharmaceutical industry is heavily regulated, and there are strict guidelines that need to be followed during clinical trials. eClinical solutions can help to ensure compliance with these regulations, leading to increased adoption.
- Growing Pressure to Reduce Costs: Clinical trials can be expensive, and eClinical solutions can help to reduce costs by streamlining processes and improving efficiency. As the pressure to reduce costs continues to grow, eClinical solutions become more attractive.
- Growing Demand for Personalized Medicine: Personalized medicine is an emerging field that requires more complex and customized clinical trials. eClinical solutions offer the flexibility to customize trials to individual patients, leading to increased adoption in this area.
To understand how our report can bring a difference to your business strategy, Inquire about a brochure at https://market.us/report/eclinical-solutions-market/#inquiry
Top trends of the eClinical Solutions market
Cloud-based eClinical solutions are becoming more and more popular, due to their scalability, cost-effectiveness, and ease of deployment. Furthermore, cloud-based systems offer improved data security and accessibility. The integration of AI and Machine Learning into eClinical solutions is growing, providing opportunities to enhance efficiency and accuracy in clinical trial data management and analysis. This includes predictive analytics, natural language processing, and image recognition. There is an increasing emphasis on patient-centricity in clinical trials, creating a unique opportunity for eClinical solution providers to create patient-centric products such as ePRO systems and mHealth applications. There is an increasing emphasis on regulatory compliance, particularly within the pharmaceutical industry. This includes using electronic signatures and audit trails to guarantee data integrity and adherence to regulatory requirements. Overall, the eClinical solutions market is rapidly transforming with an emphasis on cloud-based solutions, AI/ML technologies, patient centricity, wearable devices & IoT applications, virtual trials & regulatory compliance.
Market Growth
The rise in investments by pharmaceutical and biotechnology companies in drug development and clinical research is expected to drive the demand for eClinical solutions. These solutions help companies to improve the efficiency of clinical trials, reduce costs, and accelerate the drug development process. Overall, it is anticipated that the market for eClinical solutions will expand as more pharmaceutical and biotechnology companies realize the value of effective data management and analysis in clinical trials and as consumer demand for more sophisticated eClinical solutions rises.
Regional Analysis
Based on area analysis with a $4.20 billion market valuation, North America dominates. The Middle East and Africa (MEA) area includes Israel, Saudi Arabia, the United Arab Emirates, South Africa, Egypt, and the Philippines in addition to the rest of the world. North America has dominated the market for e-clinical solutions due to the growing target population and rising prevalence of lifestyle-related diseases like diabetes and heart conditions. Large unmet medical requirements and an increase in the prevalence of serious chronic diseases like cancer, cardiovascular problems, and communicable diseases are to blame for Asia-expected Pacific's CAGR of 15.4% from 2023 to 2030.
Have Queries? Speak to an expert or Click Here To Download/Request a Sample
Scope of the Report
| Report Attribute | Details |
| Market Value (2022) | USD 8 billion |
| Market Size (2032) | USD 27 billion |
| CAGR (from 2023 to 2032) | 12.8% |
| North America Revenue Share | 64% |
| Historic Period | 2016 to 2022 |
| Base Year | 2022 |
| Forecast Year | 2023 to 2032 |
Market Drivers
With the increasing complexity of clinical trials and the need for more efficient data management, clinical trial automation has become a critical part of the drug development process. This is driving the demand for eClinical solutions, which enable the automation of key clinical trial processes. Chronic diseases, such as cancer, diabetes, and cardiovascular disease, are on the rise globally. This has led to an increase in the number of clinical trials focused on developing treatments for these diseases, which in turn is driving the demand for eClinical solutions. The drug development process is expensive and time-consuming. eClinical solutions offer a cost-effective way to streamline the drug development process, which has led to their increasing adoption by pharmaceutical companies.
Real-world data is becoming increasingly important in drug development, as it can provide insights into how drugs perform in the real world. eClinical solutions are key in collecting, managing, and analyzing real-world data from clinical trials. Advancements in technology, such as cloud computing, mobile devices, and artificial intelligence, are driving the development of more sophisticated eClinical solutions that can automate a wider range of clinical trial processes and provide more advanced analytics.
Market Restraints
Along with the drivers, the eClinical solutions market also faces some restraints, including:
- High implementation and maintenance costs: eClinical solutions require significant investment in terms of implementation and maintenance, which can be a barrier to adoption, especially for small and mid-sized pharmaceutical companies.
- Lack of standardization: There is a lack of standardization in the eClinical solutions market, which can lead to compatibility issues and increase the complexity of the drug development process.
- Data security concerns: Clinical trial data is highly sensitive and confidential, and there is a risk of data breaches or unauthorized access to data. This can create concerns around data security and privacy, which can impact the adoption of eClinical Solutions.
Market Opportunities
The eClinical solution market offers numerous opportunities for growth and innovation. Some of the key opportunities in this market include:
- Increasing demand for efficient clinical trial management: As the number of clinical trials continues to increase, the demand for efficient clinical trial management solutions is also on the rise. eClinical solutions offer an integrated approach to managing clinical trials, including data collection, analysis, and reporting, which can help improve efficiency and reduce costs.
- Advancements in technology: With the rapid advancements in technology, eClinical solutions are becoming more sophisticated and offer a wide range of features and functionalities. These include electronic data capture, clinical trial management systems, and electronic patient-reported outcomes.
- Rising adoption of cloud-based solutions: Cloud-based eClinical solutions are becoming increasingly popular due to their ease of use, scalability, and cost-effectiveness. Cloud-based solutions also offer real-time data access, which can help improve decision-making and accelerate clinical trial timelines.
Grow your profit margin with Market.us - Purchase This Premium Report at https://market.us/purchase-report/?report_id=100469
Report Segmentation of the eClinical Solutions Market
Product Insight
Electronic Clinical Outcome Assessment (eCOA) dominates the eclinical industry, according to product analysis. The different market segments for eClinical include the Clinical Analytics Platform, Clinical Data Integration Platform, Clinical Trial Management Systems (CTMS), Electronic Clinical Outcome Assessment (eCOA), Electronic Data Capture (EDC), Clinical Data Management Systems (CDMS), Electronic Trail Master Files (eTMF), Randomization and Trial Supply Management (RTMS), Safety Solutions, and Others. The CTMS group, which in the past brought in the most money, held sway over the market for eClinical solutions. eCOA is anticipated to advance most quickly during the anticipated era as a result of the expanding significance of high-quality clinical data. The inclusion of eCOA in the measurement of patient-reported, clinician-reported, and observer-reported outcomes is growing, which helps to maintain total quality.
Mode of Deliver Insight
The eClinical market is segmented into three groups based on the delivery channel: cloud-based, licensed enterprise, and web-hosted (on-demand) (SaaS). Due to these goods' greater interoperability, the web-hosted sector dominated the market in 2022 with a market share of $2973.24 million. The cloud-based category is expected to experience the highest CAGR of 13.4% during the projection period for eclinical solutions due to its flexibility, broad accessibility, low handling costs, and straightforward data backup. The web-hosted category, which produced the largest income share throughout history, dominated the market for eClinical solutions. Suppliers can customize the display of information for different user groups thanks to the simplicity of customization, accessibility, and usability of web-hosted products. Additionally, these goods are more interoperable.
Development Phase Insight
eClinical solutions market in 2022 was dominated by phase III clinical trials, according to development phase analysis, as more pharmaceuticals effectively advanced to phase III. The phase I sector is expected to experience the highest CAGR of 13.0% over the course of the projection period due to the significant role that these systems play in forecasting future outcomes and eliminating drug candidates with the lowest probability of success. The market categories for eClinicals are Phase I, Phase II, Phase III, and Phase IV. The phase III category previously dominated the eClinical solutions industry and was responsible for the largest revenue share. The efficacy of a drug is investigated in Phase III.
End User Analysis
Through end-user research, North America is anticipated to hold the largest market share, followed by Europe, Asia-Pacific, and LAMEA, thanks to its developed business infrastructure, cutting-edge technology, and accessibility of key participants. Asia-Pacific is predicted to have the highest CAGR over the projected period due to the region's rapid technological advancement in the healthcare industry and the increase in public-private investment in the market for eClinical solutions.
Recent Development of the eClinical Solutions Market
In 2021, Signiant Health expanded its electronic informed consent options and powers with the launch of SmartSignalsTM eConsent. Due to modifications made to important product features and tiered licensing options, sponsors now have more control over obtaining electronic informed consent and re-consent of any study design. The most current version of the Bright Clinical Data CloudTM, made available in 2021 by eClinical Solutions LLC, offers improved automation of data review and mapping capabilities across the platform, stronger analytics, and more visualizations.
For more insights on the historical and Forecast market data from 2016 to 2032 - download a sample report at https://market.us/report/eclinical-solutions-market/request-sample/
Market Segmentation
Based on Product:
- Electronic Data Capture (EDC)9
- Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Electronic Clinical Outcome Assessment (eCOA)
- Randomization and Trial Supply Management Solution (RTMS)
- Safety Solutions
- Analytics and Reporting Platforms
- Integration Platforms
- Electronic Trial Master File (eTMF)
Based on Delivery Mode:
- Web-hosted (On-Demand)
- Licensed Enterprise (On-premise)
- Cloud-based/software-as-a-service (SaaS)
Based on the Development Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
Based on End-User:
- Academic Institutes
- Medical Device Manufactures
- Hospitals
- CROs
- Pharmaceutical & Biotechnology Companies
By Geography
- North America
-
- The US
- Canada
- Mexico
- Western Europe
-
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
-
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
-
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
-
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Competitive Landscape
The eClinical solution market is a rapidly growing sector in the healthcare industry, driven by the increasing demand for innovative technologies that improve patient outcomes, streamline clinical trial processes, and reduce costs. Here is an overview of the competitive landscape of the eClinical solution market:
- Oracle Corporation
- Medidata Solutions, Inc.
- Parexel International Corporation
- BioClinica, Inc.
- Signant Health
- Datatrak International, Inc.
- ERT
- eClinical Solutions, Inc.
- MaxisIT Inc.
- Bio-Optronics, Inc.
- Dassault Systemes
- IBM Watson Health
- Anju Life Sciences Software
- Merge Healthcare Incorporated
- OmniComm System
- Other Key Players
Browse More Related Reports:
- Electronic clinical outcome assessment solutions market was valued at USD 12,900 million in 2021. It is forecast to grow at a compound annual growth rate (CAGR), of 15.6% between 2022-2023.
- Preclinical imaging market size was valued at USD 3.25 billion in 2021 and is anticipated to expand at a compound annual growth rate (CAGR) of 3.8% from 2022 to 2030
- Clinical trials market size is expected to be worth around USD 886.5 billion by 2032 from USD 450.1 billion in 2022, growing at a CAGR of 7.2% during the forecast period from 2022 to 2032.
- Clinical nutrition market size is expected to be worth around USD 85.2 billion by 2032 from USD 47.4 billion in 2022, growing at a CAGR of 6.20% during the forecast period from 2022 to 2032.
About Us:
Market.US (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. Market.US provides customization to suit any specific or unique requirement and tailor-makes reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us on LinkedIn | Facebook | Twitter
Our Blog:
