Biodexa Pharmaceuticals PLC
(“Biodexa” or the “Company”)
Biodexa CEO Issues Shareholder Letter Highlighting Progress in 2023
and Expected Milestones in 2024
CARDIFF, United Kingdom, Jan. 22, 2024 (GLOBE NEWSWIRE) – Biodexa Pharmaceuticals PLC (Nasdaq: BDRX), a clinical stage biopharmaceutical company developing a pipeline of innovative products for the treatment of diseases with unmet medical needs including Type 1 diabetes and rare / orphan brain cancers, today announced that Stephen Stamp, CEO and CFO of Biodexa, has issued a Shareholder Letter highlighting the Company’s progress in 2023 and expected milestones in 2024. The full text of the letter follows:
Dear Shareholders,
I thought now would be a good time to review our Company’s progress in 2023 and our plans and expected milestones for 2024, the latter being set out in the following graphic:
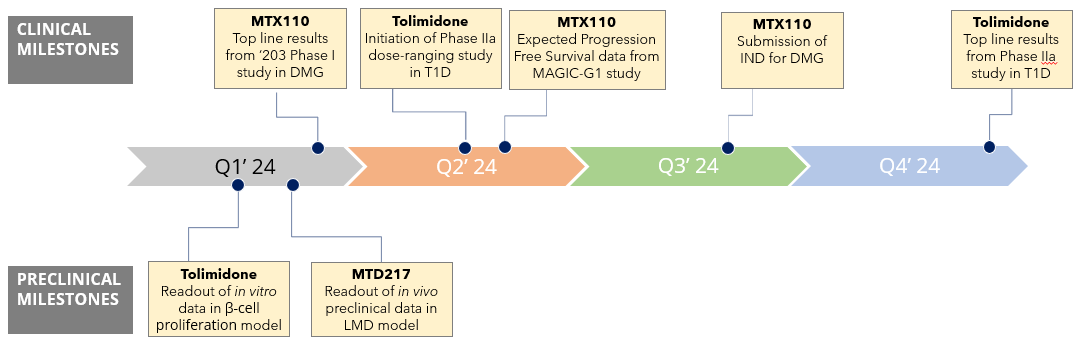
Last year proved to be a year of transition for our Company. We started the year as dual-listed drug delivery company called Midatech Pharma with a single clinical asset and we finished the year as a NASDAQ-listed, multi-asset therapeutics company re-named Biodexa Pharmaceuticals. In December 2023, we completed an in-licensing of a new, potentially disease modifying, orally delivered clinical stage molecule for Type I diabetes, an unmet medical disease. Outside of the recent FDA approval of Tzield®, developed by Provention Pharma and subsequently acquired by Sanofi (NASDAQ: SNY) for $2.9 billion, there have been limited options for the treatment of Type 1 diabetes other than exogenous insulin. Tzield® has a different, but potentially complementary, mode of action to tolimidone.
Looking at our programs one by one ….
TOLIMIDONE
We were delighted to finish the year with the in-licensing of tolimidone, a Phase II ready asset which we plan to develop for Type 1 diabetes, a genetically-driven disease where an autoimmune reaction destroys the pancreatic β-cells which produce the insulin that regulates plasma glucose levels. Originally discovered by Pfizer, it comes with an extensive preclinical and toxicology data package and has been exposed to over 700 patients, including in Type 2 diabetes. Tolimidone is an activator of lyn kinase which has been shown to play a significant role in cell proliferation, differentiation, apoptosis, migration and metabolism. We have already commissioned experiments at a CRO and expect to announce data from an in vitro study of β-cell survival and proliferation in a validated model in the first quarter of 2024.
We are also in the process of setting up a Phase IIa study of tolimidone in approximately 16 patients with Type 1 diabetes. The study will be open-label with three tolimidone doses tested in parallel. The study will include measurement of C-peptide (a marker of insulin), HbA1c (a measure of plasma glucose levels) and number of hyperglycemic events. We expect to be able to announce preliminary data before the end of 2024.
MTX110
Recurrent Glioblastoma Multiforme
Our unique formulation of panobinostat in combination with Convection Enhanced Delivery direct to the tumor continued to advance in the clinic. Because we saw no drug-related adverse events during dose escalation, we were able to recruit the minimum number in the first cohort of patients in our Phase I study (called the MAGIC-G1 study) in recurrent glioblastoma multiforme, or GBM. GBM is the most common form of adult brain cancer with incidence of 3 -4 per 100,0001 and overall survival of 13 to 30 months depending on numerous factors, including MGMT methylation2. GBM universally recurs and, once recurred, median overall survival is approximately 6.5 months3. We expect to announce Progression Free Survival data on the first cohort of patients in mid-2024.
Diffuse Midline Glioma (formerly categorized as Diffuse Intrinsic Pontine Glioma, or DIPG)
Diffuse Midline Glioma, or DMG, is a fatal pediatric brain cancer with approximately 1,100 diagnoses per annum globally4 and overall survival of 8 to 10 months5. Building on our first Phase I study in DMG, which showed median overall survival of 26 months, recruitment of a second Phase I study at Columbia University was completed in mid-2023. We expect publication of results at a major conference around the end of the first quarter of 2024. Based on the combined results of the two Phase I studies, we will evaluate the potential for an IND for what could prove a pivotal Phase II study towards the end of the year.
MTD217
When under stress from chemotherapy, to generate energy, cancer cells often switch from aerobic glycolysis pathway to an alternative oxidative phosphorylation, or OXPHOS, pathway. In 2023 we initiated a preclinical program to explore the simultaneous inhibition of both aerobic and OXPHOS pathways and we have already been able to demonstrate up to a six-fold synergistic effect of co-administering MTX110 with an OXPHOS inhibitor in three patient-derived cells lines. We have also established new patent positions to protect these combinations. Our initial target for MTD217 is leptomeningeal disease, or LMD, a lethal complication in which metastatic cancer cells, most commonly from breast and lung tumors, invade the cerebrospinal fluid and central nervous system. Approximately 5% of all cancer patients develop LMD6 and, with no effective treatments currently available, median overall survival is just three to six months post- diagnosis6. In the fourth quarter of 2023 we set up and validated a mouse LMD model. We expect to report data from the in vivo LMD efficacy model by the end of the first quarter of 2024. If positive, we aim to open an IND before year end 2024 and start a first clinical study by the end of the first quarter of 2025.
OUTLOOK
Including the $6.0 million we raised in December 2023, we have the resources to deliver two sets of preclinical data and three sets of clinical data in 2024. Our development pipeline now includes five programs, of which four are at clinical stage. With the pipeline stronger than it has ever been, we are enthusiastic about the potential for our Company in 2024 and beyond.
Sincerely,
/s/ Stephen Stamp
CEO, CFO
Forward Looking Statements
Certain statements in this shareholder letter may constitute “forward-looking statements” within the meaning of legislation in the United Kingdom and/or United States. Such statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are based on management’s belief or interpretation. All statements contained in this announcement that do not relate to matters of historical fact should be considered forward-looking statements. In certain cases, forward-looking statements can be identified by the use of words such as “plans”, “expects” or “does not anticipate”, or “believes”, or variations of such words and phrases or statements that certain actions, events or results “may”, “could”, “would”, “might” or “will be taken”, “occur” or “be achieved.” Examples of forward-looking statements include, among others, statements we make regarding our pre-clinical data and clinical trials. Forward-looking statements and information are subject to various known and unknown risks and uncertainties, many of which are beyond the ability of the Company to control or predict, that may cause their actual results, performance or achievements to be materially different from those expressed or implied thereby, and are developed based on assumptions about such risks, uncertainties and other factors set out herein.
Reference should be made to those documents that the Company shall file from time to time or announcements that may be made by the Company in accordance with the rules and regulations promulgated by the United States Securities and Exchange Commission, which contain and identify other important factors that could cause actual results to differ materially from those contained in any projections or forward-looking statements. These forward-looking statements speak only as of the date of this announcement. All subsequent written and oral forward-looking statements by or concerning the Company are expressly qualified in their entirety by the cautionary statements above. Except as may be required under relevant laws in the United States, the Company does not undertake any obligation to publicly update or revise any forward-looking statements because of new information, future events or events otherwise arising.
- Cancers | Free Full-Text | Epidemiology of Glioblastoma Multiforme; Literature Review (mdpi.com)
- Radke et al (2019). Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathologica Communications 1:89
- J Neurooncol. 2017; 135(1): 183–192
- DIPG International Registry
- DIPG.org/facts/dipg-survival-rate-and-prognosis
- https://my.clevelandclinic.org/health/diseases/22737-leptomeningeal-disease
For more information, please contact:
Biodexa Pharmaceuticals PLC |
| Stephen Stamp, CEO, CFO |
| Tel: +44 (0)29 20480 180 |
| www.biodexapharma.com |
Edison Group (US Investor Relations) |
| Laine Yonker |
| Tel: +1 (610) 716 2868 |
| Email: lyonker@edisongroup.com |
About Biodexa Pharmaceuticals PLC
Biodexa Pharmaceuticals PLC (listed on NASDAQ: BDRX) is a clinical stage biopharmaceutical company developing a pipeline of innovative products for the treatment of diseases with unmet medical needs. The Company’s lead development programmes include tolimidone, under development as a novel agent for the treatment of type 1 diabetes and MTX110, which is being studied in aggressive rare/orphan brain cancer indications, and
Tolimidone is an orally delivered, potent and selective inhibitor of lyn kinase. Lyn is a member of the Src family of protein tyrosine kinases, which is mainly expressed in hematopoietic cells, in neural tissues, liver, and adipose tissue. Tolimidone demonstrates glycemic control via insulin sensitization in animal models of diabetes and has the potential to become a first in class blood glucose modulating agent.
MTX110 is a solubilised formulation of the histone deacetylase (HDAC) inhibitor, panobinostat. This proprietary formulation enables delivery of the product via convection-enhanced delivery (CED) at chemotherapeutic doses directly to the site of the tumour, by-passing the blood-brain barrier and potentially avoiding systemic toxicity.
Biodexa is supported by three proprietary drug delivery technologies focused on improving the bio-delivery and bio-distribution of medicines. Biodexa’s headquarters and R&D facility is in Cardiff, UK. For more information visit www.biodexapharma.com.
