New York, USA, May 07, 2024 (GLOBE NEWSWIRE) -- Retinitis Pigmentosa Clinical Trial Pipeline Insights Featuring 40+ Companies | DelveInsight
Retinitis pigmentosa is a genetic disorder characterized by progressive degeneration of the retina, leading to gradual vision loss and potential blindness. The market for retinitis treatments is expanding rapidly due to factors such as changing demographics, more awareness, and a rise in the prevalence of the condition globally. Growing age demographics in the population are driving an increasing need for retinitis treatment options. Increased knowledge of the potential consequences of untreated retinitis, such as blindness, is encouraging more people to seek diagnosis and treatment, which is supporting market growth.
DelveInsight’s 'Retinitis Pigmentosa Pipeline Insight 2024' report provides comprehensive global coverage of pipeline retinitis pigmentosa therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the retinitis pigmentosa pipeline domain.
Key Takeaways from the Retinitis Pigmentosa Pipeline Report
- DelveInsight’s retinitis pigmentosa pipeline report depicts a robust space with 40+ active players working to develop 40+ pipeline therapies for retinitis pigmentosa treatment.
- Key retinitis pigmentosa companies such as MeiraGTx, Aldeyra Therapeutics, Inc., SparingVision, Frontera Therapeutics, ProQR Therapeutics, Bionic Sight, Endogena Therapeutics, Nacuity Pharmaceuticals, Kiora Pharmaceuticals, jCyte, Ocugen, Neurotech USA, Nanoscope Therapeutics, ONL Therapeutics, PulseSight Therapeutics, ViGeneron, Beacon Therapeutics, and others are evaluating new retinitis pigmentosa drugs to improve the treatment landscape.
- Promising retinitis pigmentosa pipeline therapies such as Botaretigene sparoparvovec, ADX-2191, SPVN-06, FT-002, Ultevursen, BS 01, EA-2353, NPI-001, KIO-301, Retinal stem cell therapy, OCU400, NT-501, MCO-010, ONL 1204, PST 611, VG 901, AGTC-501, and others are under different phases of retinitis pigmentosa clinical trials.
- In April 2024, Ocugen announced that the US Food and Drug Administration (FDA) has cleared the Company’s Investigational New Drug (IND) amendment to initiate a Phase III clinical trial of OCU400, a modifier gene therapy product candidate being developed for retinitis pigmentosa (RP).
- In April 2024, ViGeneron announced that the first patient had been dosed in its Phase Ib clinical trial evaluating intravitreal injection of VG901 to treat retinitis pigmentosa (RP) caused by mutations in the CNGA1 gene.
- In March 2024, Nanoscope Therapeutics announced positive top-line results after the completion of the 2-year Phase IIb RESTORE randomized, controlled clinical trial of its lead program, MCO-010, mutation-agnostic gene therapy for patients with permanent and severe vision loss from advanced retinitis pigmentosa (RP).
- In February 2024, jCyte announced the successful outcome of its pre-phase III Type B meeting with the US Food and Drug Administration (FDA) held on January 16, 2024. Additionally, the company is gearing up to commence its pivotal US trial for jCell in the second half of 2024.
- In December 2023, Ocugen announced that the FDA had granted RMAT designation to Ocugen’s investigational product OCU400 for the treatment of retinitis pigmentosa (RP) associated with RHO mutations.
- In October 2023, Kiora Pharmaceuticals received Investigational New Drug Application approval in Australia to enroll up to six additional patients in the ABACUS study of KIO-301. ABACUS was initially designed to evaluate patients with Retinitis Pigmentosa (RP).
Request a sample and discover the recent advances in retinitis pigmentosa treatment drugs @ Retinitis Pigmentosa Pipeline Report
The retinitis pigmentosa pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage retinitis pigmentosa drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the retinitis pigmentosa clinical trial landscape.
Retinitis Pigmentosa Overview
Retinitis pigmentosa refers to a collection of genetic, progressive retinal disorders leading to the gradual deterioration of the retina. It can be inherited through autosomal recessive, autosomal dominant, or X-linked recessive traits. Individuals are born with this condition, typically experiencing initial symptoms in childhood and often facing significant vision loss over time. While there is currently no cure for retinitis pigmentosa, individuals can benefit from vision aids and training programs aimed at optimizing their remaining vision. Retinitis pigmentosa diagnosis involves tests such as electroretinography (ERG), visual field testing, retinal imaging, fundus auto-fluorescence (FAF), and genetic testing.
Other diagnostic tools include Optical Coherence Tomography (OCT) and genetic testing. Unfortunately, there is no treatment available to halt the progression of RP or restore lost vision. The management of retinitis pigmentosa encompasses various approaches, including the use of dietary supplements, ozone therapy, and surgeries. Research is actively focusing on gene therapy as a potential treatment avenue for retinitis pigmentosa. Additionally, managing retinitis pigmentosa involves utilizing low-vision aids and assistive technologies. These tools include a variety of magnifiers and devices capable of identifying objects or individuals when pointed at, as well as strategies such as wearing sunglasses and limiting exposure to excessive light.
Find out more about retinitis pigmentosa treatment drugs @ Drugs for Retinitis Pigmentosa Treatment
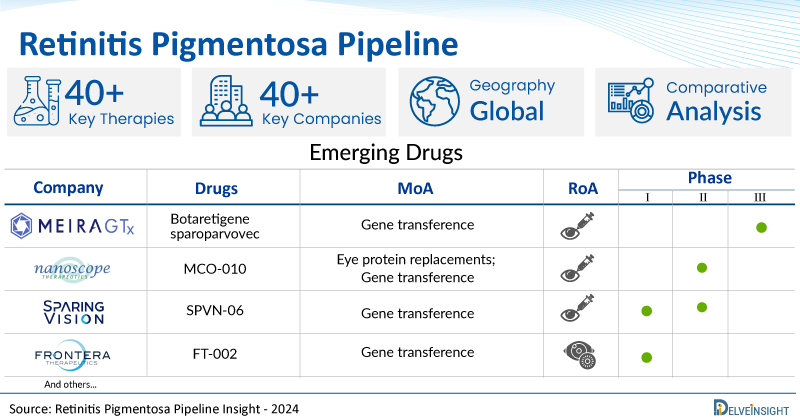
A snapshot of the Retinitis Pigmentosa Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Botaretigene sparoparvovec | MeiraGTx | Phase III | Gene transference | Subretinal |
| ADX-2191 | Aldeyra Therapeutics, Inc. | Phase II | Antimetabolites; Immunosuppressants; Tetrahydrofolate dehydrogenase inhibitors; Thymidylate synthase inhibitors | Intravitreal |
| MCO-010 | Nanoscope Therapeutics | Phase II | Eye protein replacements; Gene transference | Intravitreal |
| SPVN-06 | SparingVision | Phase I/II | Gene transference | Subretinal |
| FT-002 | Frontera Therapeutics | Phase I | Gene transference | Intraocular |
Learn more about the emerging retinitis pigmentosa pipeline therapies @ Retinitis Pigmentosa Clinical Trials
Retinitis Pigmentosa Therapeutics Assessment
The retinitis pigmentosa pipeline report proffers an integral view of the retinitis pigmentosa emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Retinitis Pigmentosa Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravitreal, Subretinal, Topical
- Therapeutics Assessment By Molecule Type: Vaccines, Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Gene transference, Antimetabolites, Immunosuppressants, Tetrahydrofolate dehydrogenase inhibitors, Thymidylate synthase inhibitors, Eye protein replacements
- Key Retinitis Pigmentosa Companies: MeiraGTx, Aldeyra Therapeutics, Inc., SparingVision, Frontera Therapeutics, ProQR Therapeutics, Bionic Sight, Endogena Therapeutics, Nacuity Pharmaceuticals, Kiora Pharmaceuticals, jCyte, Ocugen, Neurotech USA, Nanoscope Therapeutics, ONL Therapeutics, PulseSight Therapeutics, ViGeneron, Beacon Therapeutics, and others
- Key Retinitis Pigmentosa Pipeline Therapies: Botaretigene sparoparvovec, ADX-2191, SPVN-06, FT-002, Ultevursen, BS 01, EA-2353, NPI-001, KIO-301, Retinal stem cell therapy, OCU400, NT-501, MCO-010, ONL 1204, PST 611, VG 901, AGTC-501, and others
Dive deep into rich insights for new drugs for retinitis pigmentosa treatment, visit @ Retinitis Pigmentosa Drugs
Table of Contents
| 1. | Retinitis Pigmentosa Pipeline Report Introduction |
| 2. | Retinitis Pigmentosa Pipeline Report Executive Summary |
| 3. | Retinitis Pigmentosa Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Retinitis Pigmentosa Clinical Trial Therapeutics |
| 6. | Retinitis Pigmentosa Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Retinitis Pigmentosa Pipeline: Late-Stage Products (Phase III) |
| 8. | Retinitis Pigmentosa Pipeline: Mid-Stage Products (Phase II) |
| 9. | Retinitis Pigmentosa Pipeline: Early-Stage Products (Phase I) |
| 10. | Retinitis Pigmentosa Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Retinitis Pigmentosa Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Retinitis Pigmentosa Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the retinitis pigmentosa pipeline therapeutics, reach out @ Retinitis Pigmentosa Treatment Drugs
Related Reports
Retinitis Pigmentosa Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key retinitis pigmentosa companies, including Johnson & Johnson Innovative Medicine, MeiraGTx, Beacon Therapeutics, Nanoscope Therapeutics, Gensight Biologics, 4D Molecular Therapeutics, Coave Therapeutics, Ocugen, Bionic Sight, jCyte, Endogena Therapeutics, ProQR Therapeutics, Aldeyra Therapeutics, among others.
Retinitis Pigmentosa Epidemiology Forecast
Retinitis Pigmentosa Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted retinitis pigmentosa epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
X-Linked Retinitis Pigmentosa Market
X-Linked Retinitis Pigmentosa Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key X-linked retinitis pigmentosa companies including Biogen, 4D Molecular therapeutics, Applied Genetics Technology Corporation, among others.
X-Linked Retinitis Pigmentosa Pipeline
X-Linked Retinitis Pigmentosa Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key X-linked retinitis pigmentosa companies, including Biogen, 4D Molecular therapeutics, Applied Genetics Technology Corporation, among others.
Gene Therapies In Ophthalmology Competitive Landscape
Gene Therapies In Ophthalmology Competitive Landscape – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gene therapies in ophthalmology companies, including Spark Therapeutics, Regenxbio, Beacon Therapeutics, Adverum Biotechnologies, Exegenesis Bio, Frontera Therapeutics, HuidaGene Therapeutics, Nanjing IASO Biotherapeutics, GenSight Biologics, Sylentis, Neurophth Therapeutics, Johnson & Johnson Innovative Medicine, Nanoscope Therapeutics, Eyevensys, Atsena Therapeutics Inc., Coave Therapeutics, OCUGEN, INC, Visgenx, Amarna Therapeutics, Ikarovec, Homology Medicines, Ray Therapeutics, Shanghai Refreshgene Technology Co., Ltd., Complement Therapeutics, Abeona Therapeutics, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
