New York, May 22, 2024 (GLOBE NEWSWIRE) -- Market Overview:
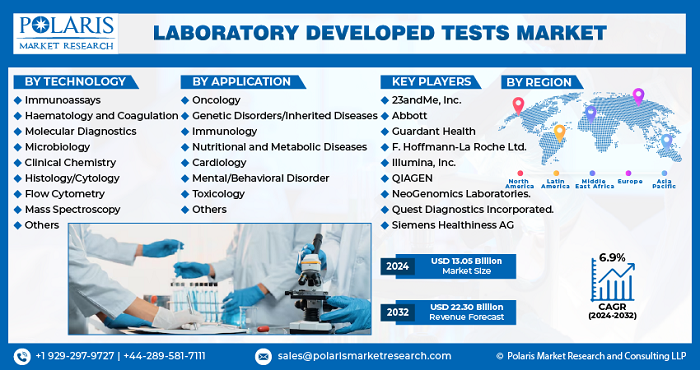
The Global Laboratory Developed Tests Market was valued at USD 12.21 billion in 2023 and is anticipated to generate an estimated revenue of USD 22.30 billion by 2032, with a CAGR of 6.9% from 2024 to 2032.
Laboratory developed tests of LDTs are in vitro diagnostic (IVDs) commodities that are deliberated for clinical usage and outlined, assembled and used within a solitary clinical laboratory that is validate under Clinical Laboratory Improvement Amendments of 1988 (CLIA) and encounters the administrative need under CLIA to accomplish escalated intricacy testing. IVDs are gadgets under the Federal Food, Drug, and Cosmetic Act (FD&C Act) involving when the assembler of the IVD is a laboratory. The rapidly rising demand for laboratory developed tests industry can be attributed to IVDs being calculated for the usage in the garnering, devising, and scrutiny of samples collected from the human body such as saliva, blood and tissue.
Moreover, the laboratory developed tests market growth can be attributed to the fact that the LDTs are advanced, confirmed, and executed within clinical laboratories such as hospital labs, individualistic reference labs, educational labs, and specific diagnostic labs. These labs must confirm administrative caliber, such as Clinical Laboratory Improvement Amendments (CLIA), to sanction standard, preciseness, and dependability of test outcomes. CLIA directives enclose a position of make-up merit that engulfs the total testing procedure, from pre-organized to organized and post-organized episodes, and involve needs for workforce limitations, quality regulation, competence testing, and lab examination.
Download Free Sample PDF Copy of the Report:
Fundamental Stats from the Report:
- The market for laboratory developed tests was valued at USD 12.21 billion in 2023.
- The market is expected to grow at a 6.9% compound annual growth rate (CAGR) from 2024-2032.
- The laboratory developed tests market size is anticipated to grow to USD 22.30 billion by 2032.
Key Findings from the Report:
- The market for laboratory developed tests is expanding due to diagnostic firms frequently cooperate with pharmaceutical firms, biotechnology organizations, and educational establishments to advance and confirm LDTs for particular illnesses or therapeutic zones.
- The market is primarily segmented by technology, application, and region.
- Europe dominated the market with the largest laboratory developed tests market share.
Request for a Discount on this Report Before Purchase:
Market Key Players:
- 23andMe, Inc.
- Abbott
- F. Hoffmann-La Roche Ltd.
- Guardant Health
- Illumina, Inc.
- NeoGenomics Laboratories.
- QIAGEN
Important Market Developments:
Growth Drivers:
- The escalating cases and pervasiveness of detrimental illnesses circumscribing conditions such as cancer, diabetes, cardiovascular illnesses, and contagious illnesses and notably impacting the demand for diagnostic testing. As the illness becomes more extensive there is a serious requirement for punctual and precise recognition to commence relevant mediation and enhance patient results. LDTs are contributory in this context aiding as an important instrument for premature illness detection, continuous observation, and customized treatment address.
- Also, LDTs provide an energetic process of recognizing the existence of detrimental illnesses at their budding stages frequently prior to the indicators present. This premature discernment sanctions healthcare donors to intervene rapidly, administer cure procedures when they are the most productive, and clearly weaken the advancement of the illnesses.
Trends:
- The forecast period will witness a sizeable growth in the laboratory developed tests market demand mainly due to LDTs playing an important part in observing the efficacy of cure and illnesses advancement over time. For patients with detrimental illnesses, systematic observation is important to estimate answers to therapy, fine-tune cure procedures as required, and recognize any untimely influences or hurdles that may emanate.
Challenges:
- Securing conformity to administrative regulations, especially Clinical Laboratory Improvement Amendments (CLIA) in the US, commands sizeable capital from labs involved in advancing and offering LDTs. To sustain CLIA credentials, labs must manifest expertise covering test advancements, authentication, quality promise, and capability testing.
Inquire more about this report before purchase:
Regional Insights:
Europe: The laboratory developed tests market in Europe is expected to escalate due to a significant rise in the affair of non transmissive illnesses strengthening the requirement for premature illness location instruments and examine productively to handle the rising pervasiveness.
North America: The region is emerging as the fastest-growing due to the escalating demand for precision medicine, which stresses customized cure policies dependent on distinct patient attributes.
Segmentation Overview:
By Technology Outlook:
- Immunoassays
- Hematology and Coagulation
- Molecular Diagnostics
- Microbiology
- Clinical Chemistry
- Histology/Cytology
- Flow Cytometry
- Mass Spectroscopy
- Others
By Application Outlook:
- Oncology
- Genetic Disorders/Inherited Diseases
- Immunology
- Nutritional and Metabolic Diseases
- Cardiology
- Mental/Behavioral Disorder
- Toxicology
- Others
By Region Outlook:
- North America (U.S., Canada)
- Europe (France, Germany, UK, Italy, Netherlands, Spain, Russia)
- Asia Pacific (Japan, China, India, Malaysia, Indonesia. South Korea, Australia)
- Latin America (Brazil, Mexico, Argentina)
- Middle East & Africa (Saudi Arabia, UAE, Israel, South Africa)
Browse More Research Reports:
About Polaris Market Research:
Polaris Market Research is a global market research and consulting company. The company specializes in providing exceptional market intelligence and in-depth business research services for PMR’s clientele spread across different enterprises. We at Polaris are obliged to serve PMR’s diverse customer base present across the industries of healthcare, technology, semiconductors, and chemicals among various other industries present around the world. We strive to provide PMR’s customers with updated information on innovative technologies, high-growth markets, emerging business environments, and the latest business-centric applications, thereby helping them always to make informed decisions and leverage new opportunities. Adept with a highly competent, experienced, and extremely qualified team of experts comprising SMEs, analysts, and consultants, we at Polaris endeavor to deliver value-added business solutions to PMR’s customers.
Contact:
Likhil G
30 Wall Street
8th Floor,
New York City, NY 10005,
United States
Phone: +1-929 297-9727
Email: sales@polarismarketresearch.com
Web: https://www.polarismarketresearch.com
Follow Us: LinkedIn | Twitter
