New York, USA, Aug. 06, 2024 (GLOBE NEWSWIRE) -- Obesity Clinical Trial Pipeline Appears Robust With 80+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
Obesity is a multifactorial disorder, which is often associated with many other significant diseases such as diabetes, hypertension and other cardiovascular diseases, osteoarthritis, and certain cancers. The Obesity market is driven by the increasing prevalence of obesity, which has heightened the demand for new and effective drugs as current treatments often fall short of managing the condition. The expanding health and wellness sector, including fitness programs and dietary supplements, aligns with the increasing focus on weight management.
DelveInsight’s 'Obesity Pipeline Insight 2024' report provides comprehensive global coverage of pipeline obesity therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the obesity pipeline domain.
Key Takeaways from the Obesity Pipeline Report
- DelveInsight’s obesity pipeline report depicts a robust space with 80+ active players working to develop 100+ pipeline therapies for obesity treatment.
- Key obesity companies such as Zealand Pharma, Sciwind Biosciences, Genexine, Sirnaomics, Sparrow Pharmaceuticals, Shionogi, Regor Pharmaceuticals, Innovent Biologics, Fractyl Health, Pfizer, NodThera Limited, Boehringer Ingelheim, Fractyl Health, TransThera, Clearmind Medicine, PegBio, Biolingus, Eli Lily &Company, Boehringer Ingelheim, Aardvark Therapeutics, Rivus Pharmaceuticals, ERX Pharmaceuticals, BioRestorative Therapies, Amolyt Pharma, Biolexis Therapeutics, Immunwork, and others are evaluating new obesity drugs to improve the treatment landscape.
- Promising obesity pipeline therapies such as ZP8396, XW003, TG103, STP705, SPI-62, S-309309, RGT001-075, IBI362, PF-06882961, NT-0796, BI-3006337, Rejuva, TT-02332, MEAI, PB 722, Liraglutide biobetter, Orforglipron, Survodutide, Bimagrumab, ARD101, HU 6, Danuglipron, ERX1000, XW017, Thermostem, AZP 3404, MLX 7006, TE 8105, and others are under different phases of obesity clinical trials.
- In June 2024, NeuroBo Pharmaceuticals announced that it had initiated the dosing of the first patient in Part 2 of its global Phase I clinical trial of DA-1726, a drug being developed for treating obesity, in the United States.
- In May 2024, Metaphore Biotechnologies joined Flagship Pioneering in announcing a research collaboration with Novo Nordisk to develop up to two next-generation therapeutics for obesity management. The collaboration is signed under the broader strategic partnership between Novo Nordisk and Flagship Pioneering to develop a portfolio of novel treatment approaches for cardiometabolic and rare diseases.
- In May 2024 Click Therapeutics, Inc., announced plans to accelerate its development initiatives in obesity and cardiometabolic disease through the acquisition of the assets of Better Therapeutics, Inc. The assets acquired include AspyreRx (BT-001) for type 2 diabetes and BT-004 for the treatment of metabolic dysfunction-associated steatohepatitis (MASH).
- In March 2024, iBio, Inc. announced that it has entered into a collaboration agreement with AstralBio, Inc. to discover, engineer, and develop novel antibodies to treat obesity and other cardiometabolic conditions.
- In February 2024, BioAge Labs, a biotech startup collaborating with Eli Lilly to trial its obesity therapy, announced that it had raised $170 million in its latest funding round.
- In January 2024, Adipo Therapeutics announced the successful completion of a $1.9 million bridge fund round. To date, Adipo has raised a total of $4 million in seed funding to advance the development of its lead asset ADPO-002 for the treatment of obesity through increased energy expenditure.
Request a sample and discover the recent advances in obesity treatment drugs @ Obesity Pipeline Report
The obesity pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage obesity drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the obesity clinical trial landscape.
Obesity Overview
Obesity involves the excessive or abnormal accumulation of fat in the body, negatively impacting health and increasing the risk of diabetes mellitus, cardiovascular disease, hypertension, and hyperlipidemia. It has become a significant public health issue, worsening over the past 50 years. Obesity is a complex condition with multiple causes, including genetic, cultural, and societal factors, and is the second leading cause of preventable death after smoking. Achieving a 5% to 10% weight loss can greatly enhance health, quality of life, and reduce economic burdens for individuals and countries. The imbalance between daily energy intake and expenditure leads to excessive weight gain, with reduced physical activity, poor diet, insomnia, endocrine disorders, and certain medications contributing to obesity. Genetic studies have highlighted the heritability of obesity, identifying numerous genes associated with increased fat and weight.
Obesity is defined as having a body mass index (BMI) over 30, while a BMI over 25 is considered overweight. Diagnosis involves assessing BMI and measuring waist size to evaluate visceral fat, along with considering related health issues like high blood pressure, cholesterol levels, and diabetes. It is the second most common preventable cause of death, linked to cardiovascular disease, diabetes mellitus, respiratory problems, psychological issues, hypertension, obstructive sleep apnea, cancer, and hyperlipidemia. Obesity rates have increased in children and adults of both genders in both developed and developing countries over the past 50 years.
Treating obesity usually requires a combination of lifestyle changes, medication, and sometimes weight-loss procedures. Lifestyle changes include adopting a healthy, reduced-calorie diet, increasing physical activity, and obtaining psychological support if needed. Medications like orlistat may be recommended when lifestyle changes are insufficient. In severe cases, weight-loss surgeries or procedures such as endoscopic sleeve gastroplasty and intragastric balloon insertion can be considered. Collaborating with healthcare professionals to set realistic weight-loss goals and maintain long-term changes is crucial for improving health and reducing obesity-related complications.
Find out more about obesity treatment drugs @ Drugs for Obesity Treatment
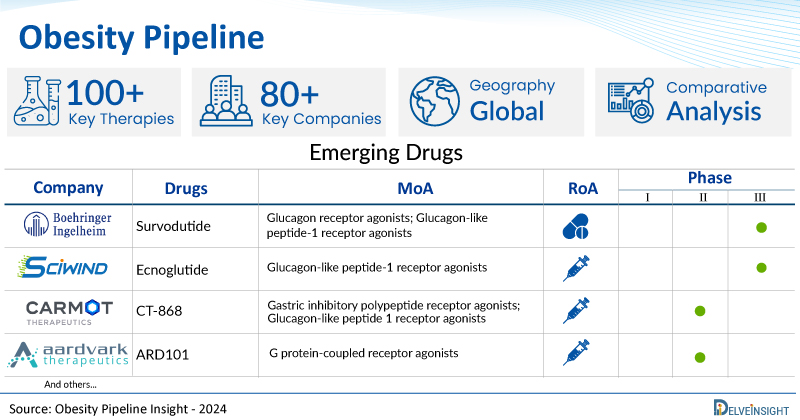
A snapshot of the Obesity Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Survodutide | Boehringer Ingelheim | Phase III | Glucagon receptor agonists; Glucagon-like peptide-1 receptor agonists | Subcutaneous |
| Ecnoglutide | Sciwind Biosciences | Phase III | Glucagon-like peptide-1 receptor agonists | Subcutaneous |
| CT-868 | Carmot Therapeutics | Phase II | Gastric inhibitory polypeptide receptor agonists; Glucagon-like peptide 1 receptor agonists | Subcutaneous |
| ARD101 | Aardvark Therapeutics | Phase II | G protein-coupled receptor agonists | Oral |
| STP705 | Sirnaomics | Phase I | Cyclo-oxygenase 2 inhibitors; Cyclooxygenase 2 expression inhibitors; RNA interference; Transforming growth factor beta1 expression inhibitors | Subcutaneous |
| BI-3006337 | Boehringer Ingelheim | Phase I | Undefined mechanism | Oral |
Learn more about the emerging obesity pipeline therapies @ Obesity Clinical Trials
Obesity Therapeutics Assessment
The obesity pipeline report proffers an integral view of the obesity emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Obesity Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Glucagon receptor agonists, Glucagon-like peptide-1 receptor agonists, Gastric inhibitory polypeptide receptor agonists, Cyclo-oxygenase 2 inhibitors, Cyclooxygenase 2 expression inhibitors, RNA interference, Transforming growth factor beta1 expression inhibitors,G protein-coupled receptor agonists.
- Key Obesity Companies: Zealand Pharma, Sciwind Biosciences, Genexine, Sirnaomics, Sparrow Pharmaceuticals, Shionogi, Regor Pharmaceuticals, Innovent Biologics, Fractyl Health, Pfizer, NodThera Limited, Boehringer Ingelheim, Fractyl Health, TransThera, Clearmind Medicine, PegBio, Biolingus, Eli Lily &Company, Boehringer Ingelheim, Aardvark Therapeutics, Rivus Pharmaceuticals, ERX Pharmaceuticals, BioRestorative Therapies, Amolyt Pharma, Biolexis Therapeutics, Immunwork
- Key Obesity Pipeline Therapies: ZP8396, XW003, TG103, STP705, SPI-62, S-309309, RGT001-075, IBI362, PF-06882961, NT-0796, BI-3006337, Rejuva, TT-02332, MEAI, PB 722, Liraglutide biobetter, Orforglipron, Survodutide, Bimagrumab, ARD101, HU 6, Danuglipron, ERX1000, XW017, Thermostem, AZP 3404, MLX 7006, TE 8105.
Dive deep into rich insights for new drugs for obesity treatment, visit @ Obesity Drugs
Table of Contents
| 1. | Obesity Pipeline Report Introduction |
| 2. | Obesity Pipeline Report Executive Summary |
| 3. | Obesity Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Obesity Clinical Trial Therapeutics |
| 6. | Obesity Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Obesity Pipeline: Late-Stage Products (Phase III) |
| 8. | Obesity Pipeline: Mid-Stage Products (Phase II) |
| 9. | Obesity Pipeline: Early-Stage Products (Phase I) |
| 10. | Obesity Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Obesity Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Obesity Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the obesity pipeline therapeutics, reach out @ Obesity Treatment Drugs
Related Reports
Obesity Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology as well as the obesity epidemiology trends.
Obesity Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key obesity companies, including Novo Nordisk, Eli Lilly and Company, CSPC Baike (Shandong) Biopharmaceutical, Jiangsu Hengrui Medicine, Carmot Therapeutics, MedImmune, Boehringer Ingelheim, Raziel Therapeutics, Pfizer, Sciwind Biosciences, Empros Pharma, Amgen, Epitomee Medical, ERX Pharmaceuticals, Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, Glaceum, Shionogi, Aardvark Therapeutics, NuSirt Biopharma, Novartis, among others.
Hypothalamic Obesity Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key hypothalamic obesity companies, including Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, among others.
Hypothalamic Obesity Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products and the key hypothalamic obesity companies, including Altimmune, Saniona, YSOPIA Bioscience, Innovent Biologics, among others.
HET Obesity/POMC Deficiency Obesity Pipeline
HET Obesity/POMC Deficiency Obesity Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products and the key HET obesity/POMC deficiency obesity companies, including Novo Nordisk, Eli Lilly, and Company, MedImmune, Boehringer Ingelheim, Raziel Therapeutics, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
