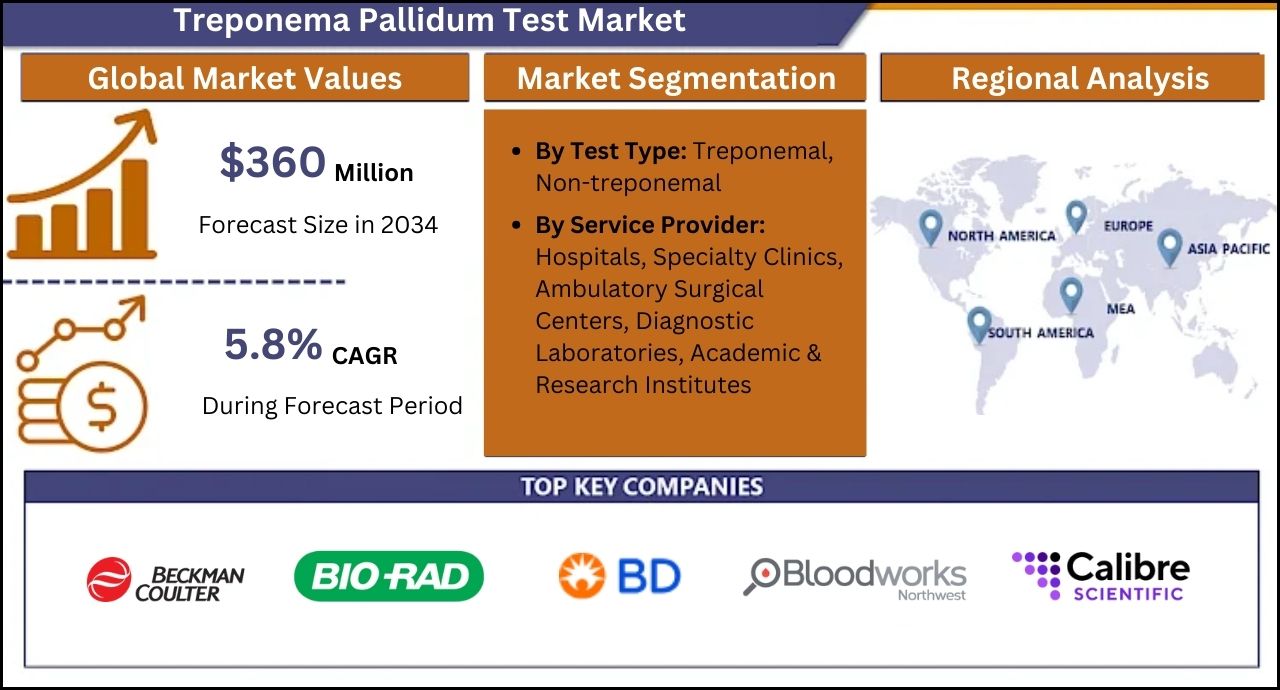
Rockville, MD, Oct. 03, 2024 (GLOBE NEWSWIRE) -- According to a new industry report released by Fact.MR, the global Treponema pallidum tests market is estimated at US$ 204.6 million in 2024 and is forecasted to reach US$ 360 million by the end of 2034. Treponemal tests (FTA, TP-PA, and EIA) are also referred to as confirmatory tests and are used to identify syphilis-specific antibodies.
Rising incidence of syphilis, caused by Treponema pallidum, has a significant impact on the demand for Treponema pallidum tests. Need for testing is driven by growing public awareness of sexually transmitted infections (STIs), particularly syphilis, and the importance of early detection through screening programs.
Demand for testing is influenced by factors that affect prevalence rates, such as evolving sexual behaviors, gaps in public health initiatives, and inadequate access to healthcare. Campaigns promoting public health, education, and advocacy are blamed for higher testing rates. The accuracy of Treponema pallidum testing is improving due to advanced molecular diagnostic techniques, point-of-care testing (POCT), and rapid diagnostic tests (RDTs). This leads to timely diagnosis and intervention. Development of novel assays and testing platforms is enhancing the efficacy of testing procedures.
For More Insights into the Market, Request a Sample of this Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=10170
Key Takeaways from the Market Study:
- The global Treponema pallidum test market is projected to expand at a CAGR of 5.8% from 2024 to 2034.
- The market in the United States is set to reach a value of US$ 37 million in 2024.
- Brazil is poised to account for 47.3% share of the market in Latin America in 2024.
- Revenue from Treponema pallidum test kit sales in Canada is set to reach US$ 5 million in 2024.
- The Latin American market is forecasted to expand at a CAGR of 6.5% from 2024 to 2034.
“Public awareness campaigns and education efforts increase knowledge about sexually transmitted infections (STIs), emphasizing the importance of routine testing for syphilis. Rapid diagnostic tests (RDTs), point-of-care testing (POCT), and molecular diagnostic techniques are improving the speed, accuracy, and accessibility of Treponema pallidum testing,” says a Fact.MR analyst.
Leading Players Driving Innovation in the Treponema Pallidum Test Market:
AdvaCare Pharma, Beckman Coulter Inc.; Bio Rad Laboratories Inc.; Becton Dickinson and Co.; bioLytical Laboratories Inc.; Bloodworks Northwest; Calibre Scientific Inc.; Danaher Corp.; OK Biotech Co. Ltd.; DiaSorin SpA; Everlywell Inc.; Hologic Inc.; LetsGetChecked; Meril Life Sciences Pvt. Ltd.; F. Hoffmann La Roche Ltd.
Hospitals Facilitating Easy Access to Treponema Pallidum Testing:
Hospitals usually offer a broad range of medical services, including diagnostic testing for different conditions. They have laboratory facilities and personnel who have been trained to perform specialized tests, such as syphilis tests. Patients seeking medical attention in hospitals have convenient access to Treponema pallidum testing in addition to other medical services.
Physicians who treat infectious diseases and specialists in laboratory medicine are among the professionals that hospitals regularly hire because they are knowledgeable in diagnosing and treating conditions like syphilis. These professionals can oversee the management of syphilis cases, ensure that test results are interpreted accurately, and provide relevant clinical guidance.
Treponema Pallidum Test Industry News:
- In March 2024, Diagnostics Direct LLC, a leading provider of diagnostic testing solutions announced the formation of its distinguished Medical Advisory Board. The Board was formed to support the organization's efforts to improve syphilis diagnosis and care and to pinpoint areas that require particular focus. The company's commitment to increasing underprivileged groups' access to Syphilis Health Check, particularly for newborns and those looking for self-testing options, was highlighted by Norman Proulx, Co-Founder and CEO.
- In January 2024, the United States Food and Drug Administration (FDA) received a de novo authorization request from NowDiagnostics for its first-to-know over-the-counter (OTC) syphilis test in the United States. The First to Know test is an at-home lateral flow immunoassay that uses a fingerstick whole-blood sample to provide results within 10 minutes. The US$ 15 million series B funding round that NowDiagnostics is currently finishing up has been made public. In addition, the company has received a strategic investment from the Labcorp Venture Fund.
Get Customization on this Report for Specific Research Solutions: https://www.factmr.com/connectus/sample?flag=S&rep_id=10170
More Valuable Insights on Offer:
Fact.MR, in its new offering, presents an unbiased analysis of the Treponema pallidum market for 2019 to 2023 and forecast statistics for 2024 to 2034.
The study divulges insights into the market based on test type (treponemal, non-treponemal) and service provider (hospitals, specialty clinics, ambulatory surgical centers, diagnostic laboratories, academic & research institutes), across seven major regions of the world (North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, and MEA).
Checkout More Related Studies Published by Fact.MR Research:
The global optical imaging agent market was valued at US$ 1,500.0 million in 2023 and has been forecasted to expand at a noteworthy CAGR of 8.1% to end up at US$ 3,533.2 million by 2034.
The global spinal fusion stimulator was valued at US$ 590.0 million in 2023 and has been forecasted to expand at a noteworthy CAGR of 6.3% to end up at US$ 1,155.4 million by 2034.
The global Chemotherapy Induced Nausea and Vomiting Treatment Market was valued at around US$ 6,412.0 million at the end of 2023. The market is projected to register a 6.4% CAGR and top a valuation of US$ 12,688.0 Million by 2034.
The global gamification mobile application in healthcare market was valued at US$ 3,895.7 million in 2023 and has been forecasted to expand at a noteworthy CAGR of 21.4% to end up at US$ 31,168.1 million by 2034.
The global heart failure monitoring systems market was valued at US$ 13,379.4 million in 2023 and has been forecasted to expand at a noteworthy CAGR of 5.7% to end up at US$ 24,612.2 million by 2034.
About Us:
Fact.MR is a distinguished market research company renowned for its comprehensive market reports and invaluable business insights. As a prominent player in business intelligence, we deliver deep analysis, uncovering market trends, growth paths, and competitive landscapes. Renowned for its commitment to accuracy and reliability, we empower businesses with crucial data and strategic recommendations, facilitating informed decision-making and enhancing market positioning. With its unwavering dedication to providing reliable market intelligence, FACT.MR continues to assist companies in navigating dynamic market challenges with confidence and achieving long-term success. With a global presence and a team of experienced analysts, FACT.MR ensures its clients receive actionable insights to capitalize on emerging opportunities and stay ahead in the competitive landscape.
Follow Us: LinkedIn | Twitter | Blog
