New York, USA, Oct. 14, 2024 (GLOBE NEWSWIRE) -- Tau Inhibitors Clinical Trial Pipeline Analysis Demonstrates 25+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
The rising incidence of Alzheimer's and other tau-related disorders, driven by an aging global population, is significantly boosting the demand for tau-targeting therapies. Innovative approaches, particularly small molecules that inhibit tau aggregation, are at the forefront of R&D. Increased funding from both government and private sectors is further accelerating advancements in neurodegenerative disease treatments, positioning tau inhibitors as a key market driver.
DelveInsight’s 'Tau Inhibitors Pipeline Insight 2024' report provides comprehensive global coverage of pipeline Tau inhibitors in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the Tau inhibitors pipeline domain.
Key Takeaways from the Tau Inhibitors Pipeline Report
- DelveInsight’s Tau inhibitors pipeline report depicts a robust space with 25+ active players working to develop 25+ pipeline Tau inhibitors.
- Key Tau inhibitor companies such as TauRx Therapeutics, Ionis Pharmaceuticals, UCB Biopharma, Alterity Therapeutics, Pinteon Therapeutics, Voyager Therapeutics, Oligomerix, Dong-A ST, Voyager Therapeutics, ADEL, Prothena, and others are evaluating new Tau Inhibitors drugs to improve the treatment landscape.
- Promising pipeline Tau inhibitors such as TRx0237, BIIB 080, Bepranemab, ATH 434, PNT-001, Research programme: gene therapies, OLX-07010, DA7503, VY7523, ADEL-Y01, PRX 005, and others are under different phases of Tau inhibitors clinical trials.
- In July 2024, Alterity Therapeutics announced positive interim data from the ATH434-202 open-label Phase II clinical trial in patients with multiple system atrophy (MSA).
- In July 2024, AC Immune Announced that its active-immunotherapy candidate, ACI-35.030 (now called JNJ-2056), targeting the pathologic form of the Tau protein, phosphorylated Tau (pTau), has received Fast Track designation from the U.S. Food and Drug Administration (FDA).
- In May 2024, Voyager Therapeutics announced that the first participants were dosed in a Phase Ia single ascending dose (SAD) trial of VY-TAU01, an investigational anti-tau antibody developed to inhibit the spread of pathological tau in Alzheimer’s disease.
- In February 2024, Oscotec and ADEL dosed the first healthy subject in a Phase Ia/Ib study of ADEL-Y01, a treatment aimed at addressing Alzheimer’s disease (AD) by targeting tau protein accumulation in the brain.
- In February 2023, Oligomerix announced the dosing of its first subjects in the company’s Phase Ia clinical trial evaluating lead candidate OLX-07010.
- In January 2023, Prothena Corporation announced positive topline Phase I single ascending dose (SAD) study results for PRX005, a potentially best-in-class investigational tri-epitopic antibody for the treatment of Alzheimer’s disease that specifically binds with high affinity to the R1, R2, and R3 repeats within the microtubule binding region (MTBR) of tau and targets both 3R and 4R tau isoforms.
Request a sample and discover the recent advances in Tau inhibitors drugs @ Tau Inhibitors Pipeline Report
The Tau inhibitors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage Tau inhibitors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the Tau inhibitors clinical trial landscape.
Tau Inhibitors Overview
Tau inhibitors are a class of compounds that target tau proteins, which are critical in maintaining the structural integrity of neurons. In diseases like Alzheimer's and other tauopathies, tau proteins become hyperphosphorylated and aggregate into neurofibrillary tangles, disrupting neuronal function and leading to cognitive decline. Tau inhibitors work by preventing the abnormal phosphorylation of tau proteins or by stabilizing their normal conformation, thereby reducing the formation of these toxic aggregates.
Research into tau inhibitors is a significant focus in neurodegenerative disease therapy due to their potential to slow or halt the progression of such conditions. These inhibitors aim to restore normal tau function and prevent the cascade of neurodegeneration associated with tau pathology. Several tau-targeting drugs are currently in clinical trials, and ongoing studies are crucial for understanding their efficacy and safety. By addressing the root cause of tau-related neurodegeneration, these therapies hold promise for improving outcomes for patients with Alzheimer's disease and other tauopathies.
Find out more about Tau inhibitor drugs @ Tau Inhibitors Analysis
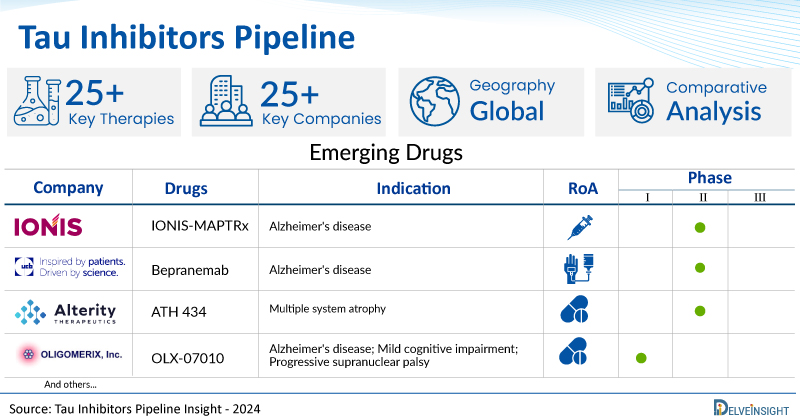
A snapshot of the Pipeline Tau Inhibitors Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| IONIS-MAPTRx | Ionis Pharmaceuticals | Phase II | Alzheimer's disease | Intrathecally |
| Bepranemab | UCB Biopharma | Phase II | Alzheimer's disease | Intravenous |
| ATH 434 | Alterity Therapeutics | Phase II | Multiple system atrophy | Oral |
| OLX-07010 | OLIGOMERIX | Phase I | Alzheimer's disease; Mild cognitive impairment; Progressive supranuclear palsy | Oral |
| VY-TAU01 | Voyager Therapeutics | Phase I | Alzheimer's disease | Intravenous |
Learn more about the emerging Tau inhibitors @ Tau Inhibitors Clinical Trials
Tau Inhibitors Therapeutics Assessment
The Tau inhibitors pipeline report proffers an integral view of the emerging Tau inhibitors segmented by stage, product type, molecule type, and route of administration.
Scope of the Tau Inhibitors Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
- Key Tau Inhibitors Companies: TauRx Therapeutics, Ionis Pharmaceuticals, UCB Biopharma, Alterity Therapeutics, Pinteon Therapeutics, Voyager Therapeutics, Oligomerix, Dong-A ST, Voyager Therapeutics, ADEL, Prothena, and others
- Key Tau Inhibitors Pipeline Therapies: TRx0237, BIIB 080, Bepranemab, ATH 434, PNT-001, Research programme: gene therapies, OLX-07010, DA7503, VY7523, ADEL-Y01, PRX 005, and others
Dive deep into rich insights for new Tau inhibitors, visit @ Tau Inhibitors Drugs
Table of Contents
| 1. | Tau Inhibitors Pipeline Report Introduction |
| 2. | Tau Inhibitors Pipeline Report Executive Summary |
| 3. | Tau Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Tau Inhibitors Clinical Trial Therapeutics |
| 6. | Tau Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Tau Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | Tau Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | Tau Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | Tau Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Tau Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Tau Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the Tau inhibitors pipeline therapeutics, reach out @ Tau Inhibitors Therapeutics
Related Reports
Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Alzheimer’s disease companies, including BioVie, AB Science, Cassava Sciences, TauRx Therapeutics, Novo Nordisk, KeifeRx, Eli Lilly, AriBio, Cerecin, Alzheon, Neurim Pharmaceuticals, Syneos Health, Athira Pharma, Annovis Bio, Anavex Life Sciences, AgeneBio, Eisai, among others.
Alzheimer's Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Alzheimer's Disease companies, including Biogen, AZTherapies, Cerecin, Neurotrope, Synaptogenix, INmune Bio, Cassava Sciences, EIP Pharma, Neuraly, AB Science, Cortexyme, Anavex Life Sciences, Athira Pharma, Time Therapeutics, Denali Therapeutics Inc., Alector Inc., Lexeo Therapeutics, TrueBinding, Inc., Vaccinex Inc., Annovis Bio Inc., Eisai Inc., Hoffmann-La Roche, Ionis Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Cognition Therapeutics, Merck Sharp & Dohme LLC, ImmunoBrain Checkpoint, AbbVie, AriBio Co., Ltd., Oryzon Genomics S.A., Eli Lilly and Company, Neurokine Therapeutics, Excelsior, Seelos Therapeutics, Inc., Janssen Research & Development, LLC, Shanghai Hengrui Pharmaceutical Co., Ltd., reMYND, Alzinova AB, VTBIO Co. LTD, BioVie Inc., Prothena Corporation plc, Coya Therapeutics, Inc., among others.
Parkinson's Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Parkinson's disease companies, including Cerevel Therapeutics, Inhibikase Therapeutics, Neuraly, Peptron, Biogen, Roche, Brain Neurotherapy Bio, Inc., Modag, Annovis Bio Inc., BioVie Inc., United Neuroscience Ltd., Luye Pharma Group, AbbVie, UCB Biopharma SRL, InnoMedica Schweiz AG, Integrative Research Laboratories AB, H. Lundbeck A/S, Shanghai WD Pharmaceutical Co., Ltd., Cerevance Beta, Inc., Nobilis Therapeutics Inc., BlueRock Therapeutics, Taiwan Mitochondrion Applied Technology Co., Ltd., among others.
Psychosis in Parkinson’s and Alzheimer’s Disease Market
Psychosis in Parkinson’s and Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key psychosis in Parkinson’s and Alzheimer’s disease companies, including Sunovion Pharmaceuticals, Karuna Therapeutics, Vanda Pharmaceuticals, Suven Life Sciences, Enterin, Intra-Cellular Therapies, Merck Sharp & Dohme, among others.
Alzheimer's Disease Epidemiology
Alzheimer's Disease Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted Alzheimer’s disease epidemiology in the 7MM, i.e., the United States, EU4 (Germany, Spain, Italy, and France), the United Kingdom, and Japan.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
