New York, USA, Oct. 15, 2024 (GLOBE NEWSWIRE) -- Prostate Cancer Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 150+ Companies Working in the Domain
Prostate cancer is one of the most common cancers among men worldwide, especially in aging populations. The increasing global elderly population leads to higher diagnosis rates, driving demand for treatment options. Additionally, advancements in treatment approaches, such as hormonal therapy, immunotherapy (like checkpoint inhibitors), targeted therapy, and next-generation androgen receptor inhibitors, are boosting the prostate cancer therapeutics market. Novel drug developments, like PARP inhibitors for metastatic castration-resistant prostate cancer (mCRPC), also contribute to market growth.
DelveInsight’s 'Prostate Cancer Pipeline Insight 2024' report provides comprehensive global coverage of pipeline prostate cancer in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the prostate cancer pipeline domain.
Key Takeaways from the Prostate Cancer Pipeline Report
- DelveInsight’s prostate cancer pipeline report depicts a robust space with 150+ active players working to develop 160+ pipeline prostate cancer drugs.
- Key prostate cancer companies such as Curium, Merck, Telix Pharmaceuticals, Exelixis, AstraZeneca, AB Science, Lantheus, Pfizer, Jiangsu Hengrui Pharmaceuticals, Modra Pharmaceuticals, Bristol-Myers Squibb, MacroGenics, Syntrix Pharmaceuticals, Zenith Epigenetics, Xencor, Bristol Myers Squibb, Merus, Phosplatin Therapeutics, Laekna Therapeutics, Tavanta Therapeutics, Madison Vaccines, Taiho Pharmaceutical, Kangpu Biopharmaceuticals, Arvinas, Candel Therapeutics, Blue Earth Therapeutics, Ipsen Biopharmaceuticals, LAVA Therapeutics, Essa Pharma, Poseida Therapeutics, Janux Therapeutics, Aurigene Oncology, Sathgen Therapeutics, Full-Life Technologies, NextPoint Therapeutics, AbbVie, SL VAXiGEN, Sorrento Therapeutics, Inc., 858 Therapeutics, Avacta Life Sciences Ltd, Nammi Therapeutics, BeiGene, DualityBio, and others are evaluating new prostate cancer drugs to improve the treatment landscape.
- Promising pipeline prostate cancer such as 177Lu-PSMA-I&T, Opevesostat (MK-5684), 177Lu-DOTA-rosopatamab, Cabozantinib, Capivasertib, Masitinib, FPI-2265, 177Lu-PNT2002, Mevrometostat (PF-06821497), Fuzuloparib, ModraDoc006, BMS-986218, Lorigerlimab, SX-682, ZEN-3694, Vudalimab, OPDIVO (nivolumab), Zenocutuzumab, Vobramitamab Duocarmazine, PT-112, LAE201, TAVT-45, pTVG-HP (MVI-816), TAS-115, KPG-121, ARV-766, CAN-2409, Saruparib (AZD5305), 177Lu-rhPSMA-10.1, Tazemetostat (Tazverik), KEYTRUDA, LAVA-1207, Masofaniten (EPI-7386), P PSMA 101, JANX 007, AUR107, MSP008-22, 225Ac-FL-020, NPX267, ABBV-969, SL-T10, Abivertinib, ETX-19477, AVA 6000, QXL138AM, BG-68501, DB-1311, and others are under different phases of prostate cancer clinical trials.
- In September 2024, Ipsen announced that the Phase III CONTACT-02 trial for Cabometyx and atezolizumab in mCRPC showed a non-significant improvement in overall survival but met the progression-free survival (PFS) endpoint.
- In August 2024, Nuvation Bio announced that the US Food and Drug Administration cleared its investigational new drug application to evaluate NUV-1511, the first clinical candidate from the company’s novel drug-drug conjugate (DDC) platform.
- In July 2024, the FDA granted fast-track designation to SYNC-T SV-102 for metastatic castrate-resistant prostate cancer (mCRPC).
- In June 2024, Kangpu Biopharmaceuticals received FDA approval for a Phase II/III trial of KPG-121 with Abiraterone for mCRPC.
- In June 2024, BioNTech SE and Duality Biologics announced that the US Food and Drug Administration granted Fast Track designation for BNT324/DB-1311 for the treatment of patients with advanced/unresectable, or metastatic castration-resistant prostate cancer (“CRPC”) who have progressed on or after standard systemic regimens.
- In May 2024, Fusion Pharmaceuticals began the Phase II AlphaBreak trial of FPI-2265 in mCRPC patients.
- In February 2024, Fusion Pharmaceuticals Inc. announced that it has entered into an exclusive worldwide license agreement with Heidelberg University and Euratom represented by the European Commission, Joint Research Centre. The license agreement grants Fusion exclusive worldwide rights to utilize, develop, manufacture, and commercialize compounds covered by the patent, which includes 225Ac-PSMA I&T for the treatment of prostate-specific membrane antigen (PSMA)-expressing cancers. In addition, Fusion and the Licensors have signed an agreement to settle the parties' dispute related to an inter partes review of a U.S. patent owned by the Licensors which was instituted in August 2023 by the United States Patent and Trademark Board.
- In February 2024, BioXcel Therapeutics, Inc. announced that the FDA granted Fast Track designation for BXCL701 with a CPI to treat metastatic small cell neuroendocrine prostate cancer (SCNC) in patients progressing on chemotherapy. This designation allows for expedited development and review by the FDA.
Request a sample and discover the recent advances in prostate cancer drugs @ Prostate Cancer Pipeline Report
The prostate cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage prostate cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the prostate cancer clinical trial landscape.
Prostate Cancer Overview
Prostate cancer is one of the most common cancers in men, primarily affecting the prostate gland, a small gland that produces seminal fluid. While the exact cause of prostate cancer is unknown, certain risk factors increase the likelihood of developing the disease. These include age (typically over 50), family history, genetic mutations (such as BRCA1/BRCA2), race (African American men are at higher risk), and lifestyle factors such as diet and obesity.
Symptoms of prostate cancer can vary and may not appear until the later stages. Common signs include difficulty urinating, weak urine flow, frequent urination (especially at night), blood in urine or semen, erectile dysfunction, and pain in the lower back, hips, or pelvis. However, many men with early-stage prostate cancer experience no symptoms at all, which is why screening is vital.
Prostate cancer diagnosis typically begins with a prostate-specific antigen (PSA) blood test and a digital rectal exam (DRE). If abnormalities are detected, further tests like a biopsy, MRI, or ultrasound may be conducted to confirm the presence and stage of cancer.
Prostate cancer treatment options depend on the stage and aggressiveness of the cancer. For early-stage prostate cancer, active surveillance may be recommended. Other treatments include surgery (prostatectomy), radiation therapy, hormone therapy, chemotherapy, and immunotherapy. In recent years, oncolytic virus therapy has emerged as a promising treatment for advanced prostate cancer, utilizing genetically engineered viruses to selectively target and destroy cancer cells.
Currently approved drugs for metastatic prostate cancer include XTANDI by Astellas/Pfizer and ZYTIGA by Janssen, both available for over a decade. Despite the introduction of ZYTIGA generics in the US in 2019 and in the EU since late 2022, causing a significant revenue drop, particularly in the US, ZYTIGA is still being actively studied in combination with emerging therapies, boosting its use with the compound abiraterone acetate.
In 2019, Janssen expanded its portfolio with ERLEADA for mHSPC, following its 2018 approval for nmCRPC. Bayer’s NUBEQA has also rapidly gained momentum, becoming a key competitor. PARP inhibitors have been making strides for patients with HRR gene mutations (BRCA1/2). AstraZeneca’s LYNPARZA was introduced for first-line prostate cancer treatment, while Pharma& Schweiz’s RUBRACA became available for 3L mCRPC patients in 2020. Additionally, in 2023, Pfizer’s TALZENNA, Janssen’s AKEEGA, and AstraZeneca/Merck’s LYNPARZA were approved for first-line use. Myovant Sciences’ ORGOVYX and Sanofi’s JEVTANA have also been approved in the US for treating mCRPC.
In 2022, Novartis' radioligand therapy, PLUVICTO, was approved for mCRPC treatment, resulting in higher-than-expected revenue in the third-line mCRPC segment. Novartis now plans to expand its presence into earlier-line mCRPC by 2024 and mHSPC by 2025 within the US market.
Find out more about prostate cancer drugs @ Prostate Cancer Analysis
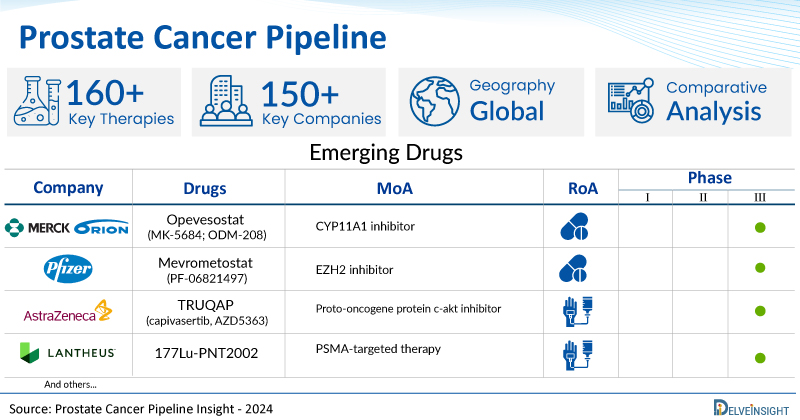
A snapshot of the Pipeline Prostate Cancer Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| 177Lu-PSMA-I&T | Curium | III | PSMA inhibitors (Prostate-specific Membrane Antigen Inhibitors) | Intravenous |
| Opevesostat (MK-5684; ODM-208) | Merck/ Orion | III | CYP11A1 inhibitor | Oral |
| Mevrometostat (PF-06821497) | Pfizer | III | EZH2 inhibitor | Oral |
| TRUQAP (capivasertib, AZD5363) | AstraZeneca | III | Proto-oncogene protein c-akt inhibitor | Intravenous |
| 177Lu-PNT2002 | Lantheus | III | PSMA-targeted therapy | Intravenous |
| 177Lu-DOTA-rosopatamab (TLX591) | Telix Pharmaceuticals | III | Ionising radiation emitter | Intravenous |
| TAVT-45 (abiraterone acetate) | Tavanta Therapeutics | III | Steroidal inhibitor of CYP17A1 | Oral |
| Saruparib (AZD5305) | AstraZeneca | III | Poly(ADP-ribose) polymerase-1 inhibitor | Oral |
| CAN-2409 (aglatimagene besadenovec) | Candel Therapeutics | III | Thymidine kinase expression stimulants | Transrectal |
| Fuzuloparib | Jiangsu Hengrui Pharmaceuticals | III | Poly(ADP-ribose) polymerase 1 inhibitors; Poly(ADP-ribose) polymerase 2 inhibitors | Oral |
Learn more about the emerging prostate cancer therapies @ Prostate Cancer Clinical Trials
Prostate Cancer Therapeutics Assessment
The prostate cancer pipeline report proffers an integral view of the emerging prostate cancer segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Prostate Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: PSMA inhibitors (Prostate-specific Membrane Antigen Inhibitors), CYP11A1 inhibitor, EZH2 inhibitor, Proto-oncogene protein c-akt inhibitor, PSMA-targeted therapy, Ionising radiation emitter, Steroidal inhibitor of CYP17A1, Poly(ADP-ribose) polymerase-1 inhibitor, Thymidine kinase expression stimulants, Poly(ADP-ribose) polymerase 2 inhibitors
- Key Prostate Cancer Companies: Curium, Merck, Telix Pharmaceuticals, Exelixis, AstraZeneca, AB Science, Lantheus, Pfizer, Jiangsu Hengrui Pharmaceuticals, Modra Pharmaceuticals, Bristol-Myers Squibb, MacroGenics, Syntrix Pharmaceuticals, Zenith Epigenetics, Xencor, Bristol Myers Squibb, Merus, Phosplatin Therapeutics, Laekna Therapeutics, Tavanta Therapeutics, Madison Vaccines, Taiho Pharmaceutical, Kangpu Biopharmaceuticals, Arvinas, Candel Therapeutics, Blue Earth Therapeutics, Ipsen Biopharmaceuticals, LAVA Therapeutics, Essa Pharma, Poseida Therapeutics, Janux Therapeutics, Aurigene Oncology, Sathgen Therapeutics, Full-Life Technologies, NextPoint Therapeutics, AbbVie, SL VAXiGEN, Sorrento Therapeutics, Inc., 858 Therapeutics, Avacta Life Sciences Ltd, Nammi Therapeutics, BeiGene, DualityBio, and others
- Key Prostate Cancer Pipeline Therapies: 177Lu-PSMA-I&T, Opevesostat (MK-5684), 177Lu-DOTA-rosopatamab, Cabozantinib, Capivasertib, Masitinib, FPI-2265, 177Lu-PNT2002, Mevrometostat (PF-06821497), Fuzuloparib, ModraDoc006, BMS-986218, Lorigerlimab, SX-682, ZEN-3694, Vudalimab, OPDIVO (nivolumab), Zenocutuzumab, Vobramitamab Duocarmazine, PT-112, LAE201, TAVT-45, pTVG-HP (MVI-816), TAS-115, KPG-121, ARV-766, CAN-2409, Saruparib (AZD5305), 177Lu-rhPSMA-10.1, Tazemetostat (Tazverik), KEYTRUDA, LAVA-1207, Masofaniten (EPI-7386), P PSMA 101, JANX 007, AUR107, MSP008-22, 225Ac-FL-020, NPX267, ABBV-969, SL-T10, Abivertinib, ETX-19477, AVA 6000, QXL138AM, BG-68501, DB-1311, and others
Dive deep into rich insights for new prostate cancer treatments, visit @ Prostate Cancer Drugs
Table of Contents
| 1. | Prostate Cancer Pipeline Report Introduction |
| 2. | Prostate Cancer Pipeline Report Executive Summary |
| 3. | Prostate Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Prostate Cancer Clinical Trial Therapeutics |
| 6. | Prostate Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Prostate Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Prostate Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Prostate Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Prostate Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Prostate Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Prostate Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the prostate cancer pipeline therapeutics, reach out @ Prostate Cancer Therapeutics
Related Reports
Prostate Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key prostate cancer companies including AstraZeneca, Arvinas, Madison Vaccines, Phosplatin Therapeutics, Hinova Pharmaceuticals, Bristol Myers Squibb, Merck, MacroGenics, Daiichi Sankyo, Seagen, Taiho Pharmaceutical, Modra Pharmaceuticals, Xencor, Point Biopharma, Lantheus Holdings, Zenith Epigenetics, Essa Pharma, Telix Pharmaceuticals, Kintor Pharmaceutical, AB Science, Eli Lilly and Company, Exelixis among others.
Metastatic Castration-Resistant Prostate Cancer Market
Metastatic Castration-Resistant Prostate Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key mCRPC companies including AstraZeneca, Merck Sharp & Dohme, Hinova Pharmaceuticals, Pfizer, Astellas Pharma, Modra Pharmaceuticals, AB Science, Eli Lilly and Company, Zr Pharma & GmbH, Bristol-Myers Squibb, Ipsen, Exelixis, Takeda, Janssen Research & Development, Tesaro, Lantheus Holdings, Kintor Pharmaceutical, MacroGenics, Daiichi Sankyo, Madison Vaccines, Novartis, Point Biopharma, Xencor, Essa Pharma, Telix International, Bayer, Arvinas, among others.
Metastatic Prostate Cancer Market
Metastatic Prostate Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key metastatic prostate cancer companies including AstraZeneca, Arvinas, Madison Vaccines, Phosplatin Therapeutics, Hinova Pharmaceuticals, Bristol Myers Squibb, Merck, MacroGenics, Daiichi Sankyo, Seagen, Taiho Pharmaceutical, Modra Pharmaceuticals, Xencor, Point Biopharma, Lantheus Holdings, Zenith Epigenetics, Essa Pharma, Telix Pharmaceuticals, Kintor Pharmaceutical, AB Science, Eli Lilly and Company, Exelixis among others.
Metastatic Castration-Sensitive Prostate Cancer Market
Metastatic Castration-Sensitive Prostate Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key mCSPC companies including Myovant Sciences, Pfizer, Bayer, Orion, Novartis, AstraZeneca, Pfizer, Janssen, Merck, Eli Lilly, among others.
Metastatic Castration-Sensitive Prostate Cancer Pipeline
Metastatic Castration-Sensitive Prostate Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key mCSPC companies, including Myovant Sciences, Pfizer, Bayer, Orion, Novartis, AstraZeneca, Pfizer, Janssen, Merck, Eli Lilly, among others.
Oncology Conference Coverage Services
DelveInsight’s Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Portfolio Management
Case Study
Learn how the engagement with respected KOLs bolstered the client's reputation as a leader in the pharma industry at KOL Profiling
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
