New York, USA, Oct. 17, 2024 (GLOBE NEWSWIRE) -- Ebola Virus Drug Development Pipeline Expands with Contributions from 18+ Key Companies | DelveInsight
The Ebola virus market is focused on developing vaccines and treatments to address outbreaks of this deadly virus. There are currently 18+ key companies involved, including major players like Auro Vaccines, INOVIO Pharmaceuticals, and RedHill Biopharma. The market is driven by the need for rapid response measures and preventive solutions to mitigate the virus's impact, particularly in regions prone to outbreaks.
DelveInsight’s 'Ebola Virus Competitive Landscape 2024' report provides comprehensive global coverage of pipeline ebola virus in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the ebola virus pipeline domain.
Key Takeaways from the Ebola Virus Pipeline Report
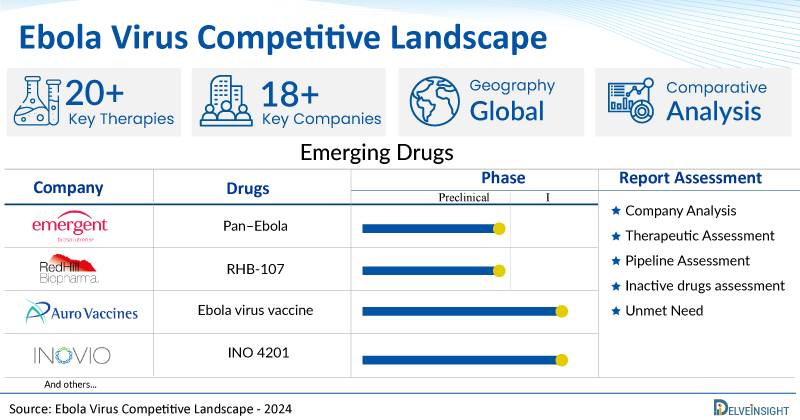
- DelveInsight’s ebola virus competitive report depicts a robust space with 18+ active players working to develop 20+ pipeline ebola virus drugs.
- Key ebola virus companies such as Auro Vaccines, INOVIO Pharmaceuticals, RedHill Biopharma Ltd., Emergent BioSolutions, Arisan Therapeutics, TFF Pharmaceuticals, China Immunotech, and others are evaluating new ebola virus drugs to improve the treatment landscape.
- Promising pipeline ebola virus such as Ebola virus vaccine, INO 4201, Opaganib, RHB-107, Pan–Ebola, ARN 75092, Research Programme: Viral vaccines, EBV TCR T cell therapy, and others are under different phases of ebola virus clinical trials.
Request a sample and discover the recent advances in ebola virus drugs @ Ebola Virus Pipeline Report
Ebola Virus Overview
The Ebola virus is a highly contagious and deadly virus that causes severe illness known as Ebola Virus Disease (EVD). It belongs to the family Filoviridae and primarily affects humans and nonhuman primates. The virus is transmitted to humans through contact with the blood, secretions, organs, or other bodily fluids of infected animals, such as fruit bats or primates. Human-to-human transmission occurs through direct contact with the body fluids of an infected person or contaminated surfaces.
The Ebola virus is primarily transmitted from animals to humans and then spreads through human-to-human contact. It is prevalent in certain regions of Africa, where outbreaks have occurred, often in areas close to rainforests. Symptoms of Ebola generally appear between 2 and 21 days after exposure. Early signs include fever, muscle pain, severe headache, and fatigue. This is followed by more severe symptoms, such as vomiting, diarrhea, rash, impaired liver and kidney function, and in some cases, internal and external bleeding. Patients may bleed from the nose, gums, or blood may appear in stool or vomit.
Diagnosing Ebola involves a combination of clinical examination and laboratory tests. Blood samples are used to detect the virus through RT-PCR, ELISA, or antigen-capture tests. Early diagnosis is crucial to control the spread of the disease. There is no specific cure for Ebola, but supportive care can significantly improve outcomes. This includes rehydration, maintaining oxygen status and blood pressure, and treating secondary infections. Experimental treatments, such as monoclonal antibodies and antiviral drugs, have shown promise. Vaccines like rVSV-ZEBOV, which was introduced during the 2014–2016 outbreak, have also been used to help control the spread of the virus.
Ebola Virus Pipeline Analysis: Drug Profile
Inmazeb: Regeneron Pharmaceuticals
Inmazeb, formerly known as REGN-EB3, was developed using Regeneron’s VelocImmune platform along with its VelociSuite technologies. The therapy consists of three structurally similar monoclonal antibodies—atoltivimab, maftivimab, and odesivimab—that target different, non-overlapping regions on the glycoprotein of Zaire ebolavirus. These antibodies work together to neutralize the Ebola virus by either preventing its entry into cells or by recruiting other immune cells to attack and eliminate infected cells.
Inmazeb is administered as a single intravenous infusion based on body weight (50 mg each of atoltivimab, maftivimab, and odesivimab per kg). Its development was supported by BARDA, part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services, under ongoing federal contracts HHSO100201700016C and HHSO100201500013C.
Find out more about ebola virus drugs @ Ebola Virus Analysis
The Ebola virus market dynamics have evolved significantly in recent years due to both the recurring outbreaks of the virus and increased global efforts to prevent future epidemics. The Ebola virus, a severe and often fatal disease, has garnered considerable attention due to its high mortality rates, with outbreaks mainly occurring in West and Central Africa. These dynamics include the demand for vaccines, treatments, diagnostics, and containment measures, which have driven substantial investments in research, development, and healthcare infrastructure. Governments, global health organizations, and pharmaceutical companies have all played a key role in the evolution of this market.
One of the primary market drivers is the repeated outbreaks of Ebola, which have spurred the development and approval of several vaccines and antiviral therapies. For example, Merck’s Ervebo vaccine, approved by the FDA in 2019, has been used in targeted vaccination campaigns in Africa. Additionally, newer therapeutics such as Regeneron’s Inmazeb and Mapp Biopharmaceutical’s ZMapp have gained regulatory approvals for treating Ebola infections. These developments have created a growing market for both vaccines and treatments, especially as stockpiling efforts by governments and international health bodies like the WHO and the CDC have intensified.
However, the Ebola virus market is also subject to several challenges and restraints. One of the key factors limiting market growth is the sporadic nature of Ebola outbreaks, making it difficult to sustain continuous demand for vaccines and treatments. In between outbreaks, attention to the virus often wanes, leading to reduced funding and investment in research and product development. Moreover, the high costs associated with vaccine production, clinical trials, and distribution in remote and under-resourced regions present significant hurdles for market players.
On the other hand, there are emerging opportunities in the Ebola virus market. One of the most promising trends is the shift toward developing broad-spectrum antiviral therapies and vaccines that can be used for multiple strains of the virus and other hemorrhagic fevers. This not only addresses the problem of strain variability but also expands the market potential beyond Ebola. Additionally, advancements in rapid diagnostic tests are likely to boost the overall market, as faster and more accurate detection of the virus will improve outbreak management and containment efforts.
To know more about the ebola virus, visit @ Ebola Virus Market Insights
A snapshot of the Pipeline Ebola Virus Drugs mentioned in the report:
| Drugs | Company | Phase |
| INO 4201 | INOVIO Pharmaceuticals | I |
| Ebola virus vaccine | Auro Vaccines | I |
| Opaganib | RedHill Biopharma Ltd. | Preclinical |
| RHB-107 | RedHill Biopharma | Preclinical |
| Pan–Ebola | Emergent BioSolutions | Preclinical |
| ARN 75092 | Arisan Therapeutics | Research |
Learn more about the emerging ebola virus therapies @ Ebola Virus Clinical Trials
Key Developments in the Ebola Virus Treatment Space
- In May 2024, RedHill announced the issue of a new Chinese patent Notice of Allowance covering opaganib as a therapy for inhibition of single-stranded RNA virus replication (notably Ebola Disease Virus) from the Chinese National Intellectual Property Administration (CNIPA), valid through 2035.
- In November 2023, SK Bioscience announced that it has signed an agreement that the company is partnering with Hilleman Laboratories Singapore to develop a low-cost, improved manufacturing process, second-generation Ebola-Zaire vaccine.
- In October 2023 RedHill Biopharma Ltd. announced that novel, twice daily, oral opaganib, delivered a statistically significant increase in survival time when given at 150 mg/kg twice a day (BID) in a United States Army Medical Research Institute of Infectious Diseases (USAMRIID) in vivo Ebola virus study, making it the first host-directed molecule to show activity in Ebola virus disease.
Scope of the Ebola Virus Pipeline Report
- Coverage: Global
- Key Ebola Virus Companies: Auro Vaccines, INOVIO Pharmaceuticals, RedHill Biopharma Ltd., Emergent BioSolutions, Arisan Therapeutics, TFF Pharmaceuticals, China Immunotech, and others
- Key Ebola Virus Pipeline Therapies: Ebola virus vaccine, INO 4201, Opaganib, RHB-107, Pan–Ebola, ARN 75092, Research Programme: Viral vaccines, EBV TCR T cell therapy, and others
Dive deep into rich insights for new ebola virus treatments, visit @ Ebola Virus Drugs
Table of Contents
| 1. | Ebola Virus Pipeline Report Introduction |
| 2. | Ebola Virus Pipeline Report Executive Summary |
| 3. | Ebola Virus Pipeline: Overview |
| 4. | Ebola Virus Marketed Drugs |
| 4.1. | Inmazeb: Regeneron Pharmaceuticals |
| 5. | Ebola Virus Clinical Trial Therapeutics |
| 6. | Ebola Virus Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Ebola Virus Pipeline: Late-Stage Products (Phase III) |
| 8. | Ebola Virus Pipeline: Mid-Stage Products (Phase II) |
| 9. | Ebola Virus Pipeline: Early-Stage Products (Phase I) |
| 9.1. | INO 4201: INOVIO Pharmaceuticals |
| 10. | Ebola Virus Pipeline: Preclinical and Discovery Stage Products |
| 10.1. | Opaganib: RedHill Biopharma Ltd. |
| 11. | Ebola Virus Pipeline Therapeutics Assessment |
| 12. | Inactive Products in the Ebola Virus Pipeline |
| 13. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 14. | Unmet Needs |
| 15. | Ebola Virus Market Drivers and Barriers |
| 16. | Appendix |
For further information on the ebola virus pipeline therapeutics, reach out @ Ebola Virus Therapeutics
Related Reports
Ebola Fever Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key ebola fever companies, including Auro Vaccines, INOVIO Pharmaceuticals, RedHill Biopharma Ltd., Emergent BioSolutions, Arisan Therapeutics, TFF Pharmaceuticals, China Immunotech, among others.
Marburg Virus Disease Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key Marburg virus disease companies, including BioCryst Pharmaceuticals, Bavarian Nordic, Flow Pharma, AlphaVax, Emergex Vaccines, ReiThera, Globavir Biosciences, Mapp Biopharmaceutical, Collaborations Pharmaceuticals, Inc., Emergent BioSolutions Inc., Auro Vaccines, Alkido Pharma, Biofactura, among others.
Marburg Virus Disease Pipeline
Marburg Virus Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key Marburg virus disease companies, including BioCryst Pharmaceuticals, Bavarian Nordic, Flow Pharma, AlphaVax, Emergex Vaccines, ReiThera, Globavir Biosciences, Mapp Biopharmaceutical, Collaborations Pharmaceuticals, Inc., Emergent BioSolutions Inc., Auro Vaccines, Alkido Pharma, Biofactura, among others.
Lassa Fever Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key Lassa fever companies, including Arisan Therapeutics, Emergent Bio Solutions, Imunon, among others.
Lassa Fever Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key Lassa fever companies, including Arisan Therapeutics, Imunon, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
