New York, USA, Oct. 28, 2024 (GLOBE NEWSWIRE) -- Complement Inhibitors Clinical Trial Pipeline Analysis: 40+ Key Companies Shaping the Future of Complement Inhibitor Therapeutics | DelveInsight
The complement inhibitors market is gaining momentum due to the rising recognition of the complement system's role in driving rare and chronic diseases, such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). As medical research uncovers the complexities of complement-related conditions, pharmaceutical companies are racing to develop targeted therapies that disrupt the underlying mechanisms. Increased diagnostic capabilities are also accelerating early detection, boosting the demand for these treatments.
DelveInsight’s 'Complement Inhibitors Pipeline Insight 2024' report provides comprehensive global coverage of pipeline complement inhibitors in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the complement inhibitors pipeline domain.
Key Takeaways from the Complement Inhibitors Pipeline Report
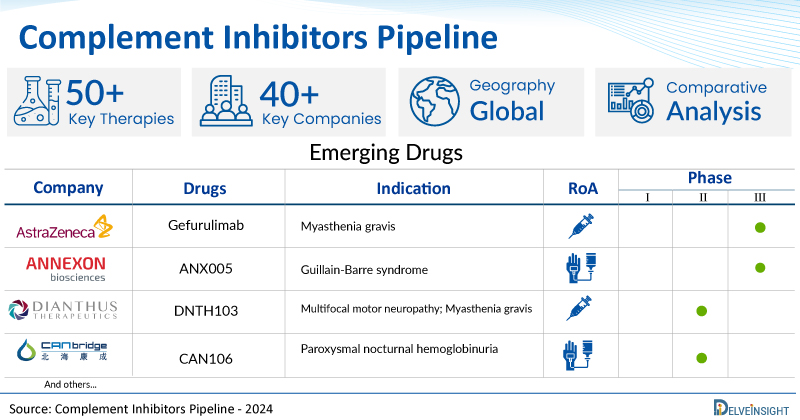
- DelveInsight’s complement inhibitors pipeline report depicts a robust space with 40+ active players working to develop 50+ pipeline complement inhibitors.
- Key complement inhibitors companies such as AstraZeneca, Annexon, Inc., Dianthus Therapeutics, Alsonex Pharmaceuticals, Mallinckrodt, CANbridge Pharmaceuticals, Beijing Defengrei Biotechnology, NovelMed Therapeutics, DynamiCure Biotechnology, CSL Behring, Kriya Therapeutics, Argenx, Arrowhead Pharmaceuticals, Amyndas Pharmaceuticals, Q32 Bio, Kira Pharmaceuticals, ReAlta Life Sciences, ISU Abxis, and others are evaluating new complement inhibitors drugs to improve the treatment landscape.
- Promising pipeline complement inhibitors such as Gefurulimab, ANX005, DNTH103, ALS-205, SLN-MNK-3, CAN106, BDB-1, NM8074, DCSZ11, KRIYA-825, Empasiprubart, ARO-C3, AMY 101, ADX-097, KP104, ISU305, RLS-0071, CSL040, and others are under different phases of complement inhibitors clinical trials.
- In October 2024, Q32 Bio announced that it will present clinical data supporting its program for innate immunity, ADX-097, at the American Society of Nephrology (ASN) Kidney Week 2024, taking place October 24-27, 2024, in San Diego, Calif.
- In September 2024, ReAlta Life Sciences announced that the first patient had been dosed in its Phase II study of RLS-0071, the Company’s lead therapeutic candidate, for the treatment of hospitalized patients with moderate to very severe steroid-refractory acute graft-versus-host disease.
- In June 2024, Annexon announced positive topline results from its randomized placebo-controlled pivotal Phase III trial in patients with Guillain-Barré syndrome.
- In June 2024, Dianthus Therapeutics announced the US Food and Drug Administration clearance of its Phase II Investigational New Drug application for the MoMeNtum trial of DNTH103 in patients with Multifocal Motor Neuropathy.
- In June 2024, NovelMed announced the US Food and Drug Administration's clearance of Ruxoprubart, the investigational drug, for commencing an efficacy trial targeting patients with ANCA Associated Vasculitis (AAV), a rare autoimmune chronic ailment characterized by inflammation in small blood vessels
- In February 2024, NovelMed announced that the US Food and Drug Administration has granted Orphan Drug Designation to Ruxoprubart, an alternative pathway (AP) blocker anti-Bb antibody, for the treatment of Paroxysmal Nocturnal Hemoglobinuria.
Request a sample and discover the recent advances in complement inhibitors drugs @ Complement Inhibitors Pipeline Report
The complement inhibitors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage complement inhibitors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the complement inhibitors clinical trial landscape.
Complement Inhibitors Overview
Complement inhibitors are a type of drug designed to target the complement system, a crucial component of the immune system responsible for inflammation, pathogen elimination, and maintaining tissue health. The complement system consists of over 30 proteins and can be activated through three pathways: classical, alternative, and lectin. Once activated, a cascade of events leads to the formation of the membrane attack complex (MAC), which can destroy both pathogens and cells. However, excessive or uncontrolled activation of the complement system is linked to several diseases, including autoimmune disorders, kidney diseases, and conditions such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Complement inhibitors work by blocking key proteins in the cascade, thereby preventing tissue damage caused by overactivation.
A well-known complement inhibitor is eculizumab, which targets C5, a protein in the terminal complement pathway. By blocking C5, eculizumab prevents the formation of the MAC and reduces the risk of complement-mediated cell destruction. This drug has shown effectiveness in treating PNH, a rare blood disorder, and aHUS, which can cause kidney damage. Newer complement inhibitors target different points in the cascade, such as C3 or Factor D, offering more specific approaches to complement inhibition. These drugs are particularly important for patients with diseases resistant to C5 inhibitors or for those who may benefit from broader inhibition upstream in the pathway.
Complement inhibitors are a rapidly expanding area of therapeutics with applications beyond rare diseases. In addition to PNH and aHUS, research is exploring their use in more common conditions like age-related macular degeneration and lupus nephritis, where complement system overactivity is a key factor. The development of new complement inhibitors is being fueled by advances in our understanding of complement biology, and with numerous clinical trials underway, the future of complement therapeutics is expected to grow considerably. This expanding field highlights the potential of complement inhibitors to meet both existing medical needs and new treatment possibilities across various diseases.
Find out more about complement inhibitors drugs @ Complement Inhibitors Analysis
A snapshot of the Pipeline Complement Inhibitors Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| Gefurulimab | AstraZeneca | III | Myasthenia gravis | Subcutaneous |
| ANX005 | Annexon, Inc. | III | Guillain-Barre syndrome | Intravenous |
| DNTH103 | Dianthus Therapeutics | II | Multifocal motor neuropathy; Myasthenia gravis | Subcutaneous |
| NM8074 | NovelMed Therapeutics | II | Paroxysmal nocturnal hemoglobinuria; IgA Nephropathy; Anti-neutrophil cytoplasmic antibody-associated vasculitis | Intravenous |
| CAN106 | CANbridge Pharmaceuticals | I/II | Paroxysmal nocturnal hemoglobinuria | Intravenous |
| TJ 210 | I-MAB Biopharma | I | Autoimmune disorders; Solid tumors | Injection |
Learn more about the emerging complement inhibitors @ Complement Inhibitors Clinical Trials
Complement Inhibitors Therapeutics Assessment
The complement inhibitors pipeline report proffers an integral view of the emerging complement inhibitors segmented by stage, product type, molecule type, and route of administration.
Scope of the Complement Inhibitors Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Key Complement Inhibitors Companies: AstraZeneca, Annexon, Inc., Dianthus Therapeutics, Alsonex Pharmaceuticals, Mallinckrodt, CANbridge Pharmaceuticals, Beijing Defengrei Biotechnology, NovelMed Therapeutics, DynamiCure Biotechnology, CSL Behring, Kriya Therapeutics, Argenx, Arrowhead Pharmaceuticals, Amyndas Pharmaceuticals, Q32 Bio, Kira Pharmaceuticals, ReAlta Life Sciences, ISU Abxis, and others
- Key Complement Inhibitors Pipeline Therapies: Gefurulimab, ANX005, DNTH103, ALS-205, SLN-MNK-3, CAN106, BDB-1, NM8074, DCSZ11, KRIYA-825, Empasiprubart, ARO-C3, AMY 101, ADX-097, KP104, RLS-0071, ISU305, CSL040, and others
Dive deep into rich insights for new complement inhibitors, visit @ Complement Inhibitors Drugs
Table of Contents
| 1. | Complement Inhibitors Pipeline Report Introduction |
| 2. | Complement Inhibitors Pipeline Report Executive Summary |
| 3. | Complement Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Complement Inhibitors Clinical Trial Therapeutics |
| 6. | Complement Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Complement Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | Complement Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | Complement Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | Complement Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Complement Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Complement Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the complement inhibitors pipeline therapeutics, reach out @ Complement Inhibitors Therapeutics
Related Reports
Complement Inhibitors Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key complement inhibitors companies, including Genentech, Ionis, Roche, AKARI Therapeutics, CARE Pharma, NovelMed Therapeutics, Omeros Corporation, among others.
Myasthenia Gravis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key myasthenia gravis companies, including Viela Bio, UCB Pharma, Momenta Pharmaceuticals, Sanofi, Regeneron Pharmaceuticals, Ra Pharmaceuticals, Hoffmann-La Roche, Alexion Pharmaceuticals, Catalyst Pharmaceuticals, Harbour BioMed, Novartis, Takeda, DAS Therapeutics, RemeGen, Cartesian Therapeutics, Nanjing IASO Biotherapeutics, Cabaletta Bio, CytoDyn, Ahead Therapeutics, Toleranzia, Rallybio, among others.
Generalized Myasthenia Gravis Pipeline
Generalized Myasthenia Gravis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key generalized myasthenia gravis companies, including Biocon, Cartesian Therapeutics, UCB, Momenta Pharmaceuticals, HanAll Biopharma, Roche, Alexion, Novartis, Takeda, BioMarin, among others.
Generalized Myasthenia Gravis Market
Generalized Myasthenia Gravis Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key generalized myasthenia gravis companies, including UCB Biopharma, Argenx-Halozyme Therapeutics, Horizon Therapeutics, Hoffmann-La Roche, Janssen Research & Development, LLC, Immunovant Sciences GmbH, Sanofi, Cartesian Therapeutics, Takeda, DAS Therapeutics, Chugai Pharmaceutical, Inc., Alexion, Regeneron Pharmaceuticals, Ra Pharmaceuticals, Inc., among others.
Warm Autoimmune Hemolytic Anemia Pipeline
Warm Autoimmune Hemolytic Anemia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key wAIHA companies, including Immunovant Sciences GmbH, Apellis Pharmaceuticals, Inc., Bioverativ, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
