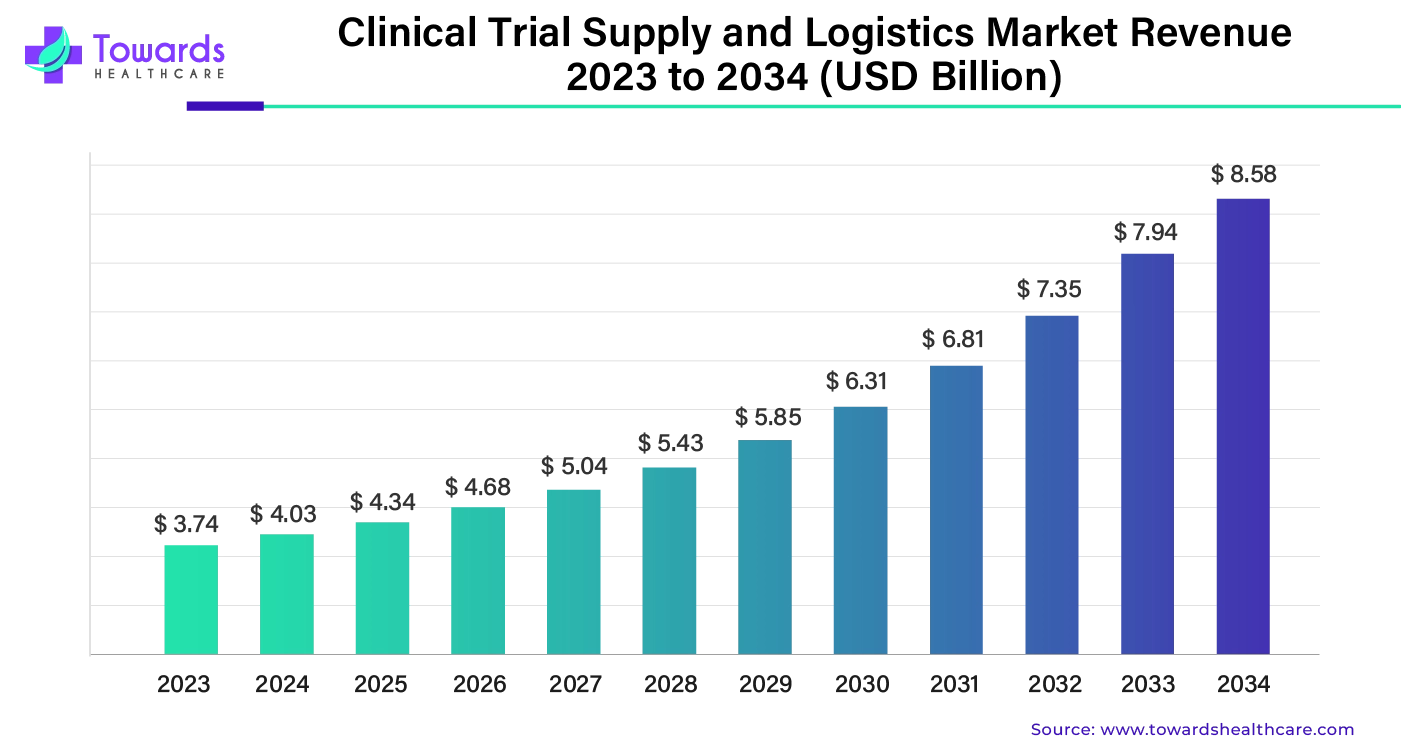
Ottawa, Nov. 21, 2024 (GLOBE NEWSWIRE) -- The global clinical trial supply and logistics market size was valued at USD 4.03 billion in 2024 and is predicted to hit around USD 7.94 billion by 2033, a study published by Towards Healthcare a sister firm of Precedence Statistics.
Download a sample version of this report @ https://www.towardshealthcare.com/download-statistics/5226
The clinical trial supply and logistics market deals with sourcing, packaging, storing, and distributing biotechnological and pharmaceutical products that are required for a clinical trial. Clinical trial supply and logistics play a role in managing drugs, equipment, and data during the clinical trial. Pharmaceutical and biotechnology companies are making efforts to outcompete each other in terms of innovation. Thus, clinical trial supply chain management is becoming important these days. Technological advancements such as blockchain and real-time tracking are expected to make the supply chain more efficient.
The rising acceptance of AI technologies is revolutionizing the market. Integrating AI can streamline and optimize several processes in pharma and biotech companies. The rapid increase in the number of clinical trials is expected to contribute to market expansion.
- According to worldwide clinical trial statistics, there were 2,784 phase I clinical drug trials initiated in 2021, compared to 2,306 phase I trials initiated during the previous year.
Major Trends in the Clinical Trial Supply and Logistics Market
- Shift Toward Decentralized Clinical Trials (DCTs): Decentralized clinical trials (DCTs) have become more popular, especially during and after the COVID-19 pandemic, which increases the trial availability. DCTs allow remote patient participation and reduce the need for physical site visits. According to Clinical Trials Arena, DCT use in 2022 was significantly higher than in 2020. The use of decentralization in Phase I trials increased by 14% in 2022 compared to 2021.
- Increasing Usage of Advanced Technologies: Advanced technologies, such as AI and blockchain, are revolutionizing the market. These technologies streamline inventory management, thereby enhancing the supply chain. According to a survey conducted by Deloitte in 2023, about 45%of clinical logistics providers had implemented the Internet of Things (IoT) connected devices. Furthermore, the use of blockchain is increasing in clinical trials among leading firms such as Thermo Fisher Scientific. The number of companies using Blockchain technology to manage clinical trials rose by 20% between 2022 and 2024 to address data integrity.
- Rising Focus on Personalized Treatment: The rising interest in a patient-centric approach is boosting the market growth. Personalized treatments such as CAR-T and gene therapy require a holistic logistics management strategy. The Global Alliance for Genomics and Health (GA4GH) recently reported that the number of personalized medicine trials rose to 40% from 2022 to 2023, focusing on the importance of cryogenic storage and temperature-controlled transport systems.
Get the latest insights on healthcare industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Regional Insights
North America’s Sustained Dominance
In 2023, North America dominated the clinical trial supply and logistics market with the largest share. This is mainly due to its well-established healthcare infrastructure and large investment in clinical trials. NIH claimed that more than 14,000 clinical trials were ongoing in the U.S. in 2023, which makes it the world leader in clinical trials. The region boasts leading pharmaceutical companies and logistics providers.
Laws such as the 21st Century Cures Act contributed to market expansion. This law is designed to boost the development of medical products and bring new innovations. Canada captured the maximum share of the market due to an increase in cancer trials. Moreover, the rising focus on precision medicine and decentralized clinical trials (DCTs) in North America bolstered the market in the region.
Asia Pacific: Fastest-growing Region
The market in Asia Pacific is expected to expand at the highest CAGR from 2023 to 2030. China is a leading contributor to regional market growth due to its diverse pool of patients and growing pharmaceutical sector. India is also another strategic market due to the rising number of phase III trials. Moreover, India focuses on trials of rare diseases and vaccines, which influence logistics strategies such as cryogenic.
Biopharmaceutical development has made South Korea one of the chosen centers of clinical trials, having set up 4300 by 2023, which is funded by MOHW.
Rising government initiatives to advance healthcare infrastructure further contribute to regional market expansion. On the other hand, relatively low trial operations costs and continuing development of healthcare facilities make the region attractive for multinational pharmaceutical corporations. According to the WHO Clinical Trials Registry, 15% of clinical trials were conducted in 2023 Asia-Pacific, as it has become an essential part of the market.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Market Segment Insights
- By service, the logistics & distribution segment held the largest share of the market in 2023. This is mainly due to the increased demand for specialized logistics and distribution services. Preference for temperature-sensitive transport and storage of biologics and precision medicines has increased in the past few years. Moreover, with the growing popularity of decentralized clinical trials, the demand for direct-to-patient delivery models increased the demand for these services.
- By phase, the phase III segment led the market with the largest share in 2023. Phase III trials enroll a large number of patients, require many tests, and necessitate precise management of supply chains to make trial supplies available on time. In 2023, phase III trials received about 40% of the clinical trial budgets worldwide, reflecting their importance in the approval of new therapies.
- By therapeutic area, the cardiovascular disorders segment dominated the market with the largest share in 2023. This is mainly due to the rapid increase in drug development as a result of the rising prevalence of cardiovascular disorders.
- By end-use, the pharmaceuticals segment led the market in 2023. Pharmaceutical companies often require trial supply and logistics services due to their continuous involvement in trials. These companies employ the services of third-party logistics service providers to coordinate complicated cross-border movements and deliveries of trial materials to different regions.
Competitive Landscape & Major Breakthroughs in the Clinical Trial Supply and Logistics Market
The clinical trial supply and logistics market experienced significant growth in 2023 and is expected to witness lot of changes due to intense competition among the key players such as Almac Group, Ltd, Catalent, Inc., Ceva Logistics, AG, Clinevo Technologies, Credevo, Experic, FedEx, IQVIA, Oximio, PAREXEL Informatics, Inc., Piramal Pharma Solutions, Proventa International, and Thermo Fisher Scientific, Inc. These firms often deal with cold chain transport, temperature-controlled storage, and decentralized trial support.
Key areas of focus of market players include:
- End-to-End Supply Chain Management: It connects all stages of production, from suppliers to consumers, to ensure that products are delivered on time, in proper condition and at the right place.
- Decentralized Trial Support: This includes delivering trial supplies to the patient’s place at the right time.
- Temperature-sensitive Logistics: Storing delicate products such as vaccines and cell therapies that have sensitive temperature variation requirements.
- Digital Transformation: It involves using AI, blockchain, and IoT to enhance production line efficiency.
Recent Development
- In February 2024, FedEx unveiled its ‘FedEx Life Science Center’ in Mumbai, setting a benchmark in the clinical trial supply chain in India and globally.
Browse More Insights of Towards Healthcare:
- The core clinical molecular diagnostics market was estimated at US$ 5.3 billion in 2023 and is projected to grow to US$ 14.45 billion by 2034, rising at a CAGR of 9.54% from 2024 to 2034.
- The clinical trial investigative site network market was estimated at US$ 8.36 billion in 2023 and is projected to grow to US$ 15.99 billion by 2034, rising at a CAGR of 6.07% from 2024 to 2034.
- The clinical microbiology market size was valued at US$ 4.61 billion in 2023 and is expected to reach US$ 7.78 billion by 2033, poised to grow at a CAGR of 5.37% from 2024 to 2033.
- The health insurance market size is calculated at US$ 1.78 trillion in 2024 and is expected to be worth US$ 3.63 trillion by 2034, expanding at a CAGR of 7.4% from 2024 to 2034.
- The digital health market size is calculated at US$ 335.51 billion in 2024 and is expected to be worth US$ 1,080.21 billion by 2034, expanding at a CAGR of 13.1% from 2024 to 2034.
- The cord blood banking services market was estimated at US$ 33.9 billion in 2023 and is projected to grow to US$ 65.36 billion by 2034, rising at a CAGR of 6.15% from 2024 to 2034.
- The drug screening market size was estimated at US$ 6.15 billion in 2023 and is projected to grow to US$ 10.34 billion by 2034, rising at a CAGR of 4.84% from 2024 to 2034.
- The pharmaceutical CDMO market size was estimated at US$ 146.05 billion in 2023 and is projected to grow to US$ 315.08 billion by 2034, rising at a CAGR of 7.24% from 2024 to 2034.
- The comparator drug sourcing market size was estimated at US$ 1.16 billion in 2023 and is projected to grow to US$ 2.24 billion by 2034, rising at a CAGR of 6.15% from 2024 to 2034.
- The physician practice management market was estimated at US$ 118.9 billion in 2023 and is projected to grow to US$ 291.7 billion by 2034, rising at a CAGR of 8.5% from 2024 to 2034.
Segments Covered in the Report
By Service
- Logistics & Distribution
- Manufacturing
- Storage & Retention
- Packaging, Labeling & Blinding
- Comparator Sourcing
- Others
By Phase
- Phase III
- Phase II
- Phase I
- Phase IV
By Therapeutic Area
- Cardiovascular Disorders
- Oncology
- Respiratory Diseases
- CNS and Mental Disorders
- Others
By End-Use
- Pharmaceuticals
- Biologicals
- Medical Devices
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa (MEA)
View in-depth Clinical Trial Supply and Logistics Market TOC: https://www.towardshealthcare.com/table-of-content/clinical-trial-supply-and-logistics-market-sizing
Acquire our comprehensive analysis today @ https://www.towardshealthcare.com/price/5226
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com
