Selbyville, Delaware, Dec. 18, 2024 (GLOBE NEWSWIRE) --
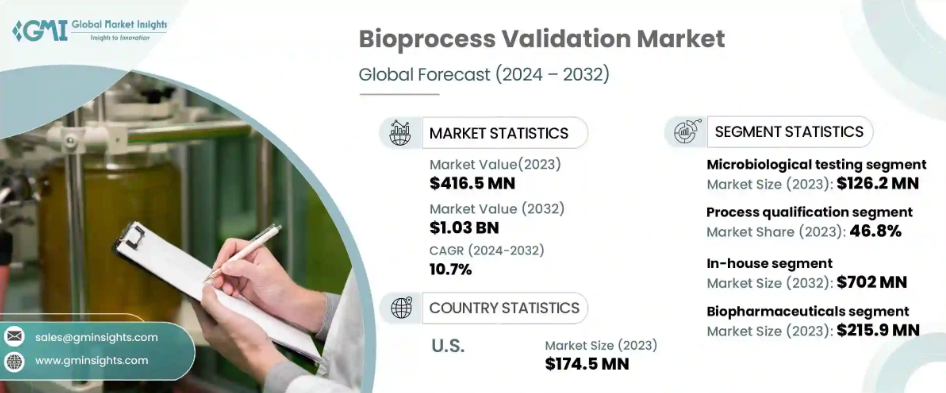
Bioprocess validation market size reached USD 416.5 million in 2023 and is anticipated to grow at 10.7% CAGR from 2024 to 2032. Bioprocess validation plays a critical role in biopharmaceutical manufacturing, ensuring that processes consistently deliver products of high quality and safety.
Request for a sample of this research report @ https://www.gminsights.com/request-sample/detail/12192
It involves systematic tests and analyses to verify that every stage, from raw material usage to final product output, meets strict regulatory and quality requirements.
The bioprocess validation market expansion is largely driven by stringent regulatory demands, rising adoption of biologics, and advancements in validation technologies. As the biopharmaceutical sector grows, both in production capacity and outsourcing, there is an increasing need for comprehensive validation protocols to maintain compliance and uphold product standards.
The surging demand for biologics, including monoclonal antibodies, vaccines, and gene therapies, further amplifies the necessity for robust validation processes, as these therapies must meet exacting standards for quality, safety, and efficacy.
Segmented by testing types, the bioprocess validation market encompasses areas such as extractables and leachables testing, microbiological testing, integrity testing, and viral clearance testing, among others. In 2023, microbiological testing emerged as a dominant segment, generating USD 126.2 million in revenue.
Regulatory bodies emphasize the importance of microbiological testing in ensuring sterility and quality throughout the manufacturing process. Adherence to Good Manufacturing Practices (GMP) and routine environmental monitoring are critical to safeguarding the integrity of biopharmaceutical products, underscoring the significance of this testing category.
By stage, the bioprocess validation market is categorized into process design, process qualification, and continued process verification. Process qualification accounted for the largest share in 2023, representing 46.8% of the market. This stage is essential in confirming that manufacturing processes consistently produce products within established parameters. By simulating real operating conditions, process qualification ensures the reliability and reproducibility of biomanufacturing protocols, bridging process design, and ongoing verification phases.
U.S. bioprocess validation market reached USD 174.5 million in 2023 and is projected to grow at a CAGR of 10.8% during the forecast period. The regulatory landscape in the U.S. mandates rigorous validation practices driven by agencies such as the FDA. Compliance with GMP standards necessitates thorough validation of all manufacturing stages, prompting increased investments in innovative validation techniques.
The growing biopharmaceutical industry, coupled with regulatory pressures and the rising prevalence of biologics, positions bioprocess validation as a cornerstone of modern healthcare manufacturing.
Request for Report Customization @ https://www.gminsights.com/roc/12192
Major players in bioprocess validation market include Asahi Kasei Corporation, Bio-Rad Laboratories, Charles River Laboratories, Danaher Corporation, Eurofins Scientific, Lonza Group, Meissner Filtration Products, Merck KGaA, Pace Analytical Services, Repligen Corporation, Sartorius AG, SGS S.A., Thermo Fisher Scientific, Tosoh Corporation, and WuXi AppTec among others.
Partial Table of Contents (ToC) of the report:
Chapter 1 Methodology & Scope
1.1 Market scope & definitions
1.2 Research design
1.2.1 Research approach
1.2.2 Data collection methods
1.3 Base estimates & calculations
1.3.1 Base year calculation
1.3.2 Key trends for market estimation
1.4 Forecast model
1.5 Primary research and validation
1.5.1 Primary sources
1.5.2 Data mining sources
Chapter 2 Executive Summary
2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
3.1 Industry ecosystem analysis
3.2 Industry impact forces
3.2.1 Growth drivers
3.2.1.1 Stringent regulations regarding safety and quality
3.2.1.2 Increasing demand for biopharmaceuticals
3.2.1.3 Rising R&D expenditure in life sciences sector
3.2.1.4 Increasing demand for bioprocess validation outsourcing
3.2.2 Industry pitfalls & challenges
3.2.2.1 Complexity and time-intensive nature of validation
3.2.2.2 High costs associated with validation processes
3.3 Growth potential analysis
3.4 Regulatory landscape
3.5 Technological landscape
3.6 Future market trends
3.7 Gap analysis
3.8 Porter’s analysis
3.9 PESTEL analysis
Browse more pharma manufacturing industry reports @ https://www.gminsights.com/industry-reports/pharma-manufacturing/82
About Global Market Insights
Global Market Insights Inc., headquartered in Delaware, U.S., is a global market research and consulting service provider, offering syndicated and custom research reports along with growth consulting services. Our business intelligence and industry research reports offer clients with penetrative insights and actionable market data specially designed and presented to aid strategic decision making. These exhaustive reports are designed via a proprietary research methodology and are available for key industries such as chemicals, advanced materials, technology, renewable energy, and biotechnology.
