United Kingdom, London, Dec. 19, 2024 (GLOBE NEWSWIRE) -- Australian healthcare technology company Resonance Health completed the acquisition of 100% of clinical research center TrialsWest for a total consideration of A$8 million ($5.20 million). The transaction marked a strategic expansion into clinical trial operations, setting the stage for future growth in the sector. The deal included an upfront cash payment of A$4 million, alongside a contingent earnout of up to A$4 million, contingent on TrialsWest achieving an EBITDA of A$1.33 million within the financial period ending FY 2026. This acquisition reinforced Resonance Health’s commitment to broadening its capabilities and enhancing its position in the clinical trials market.
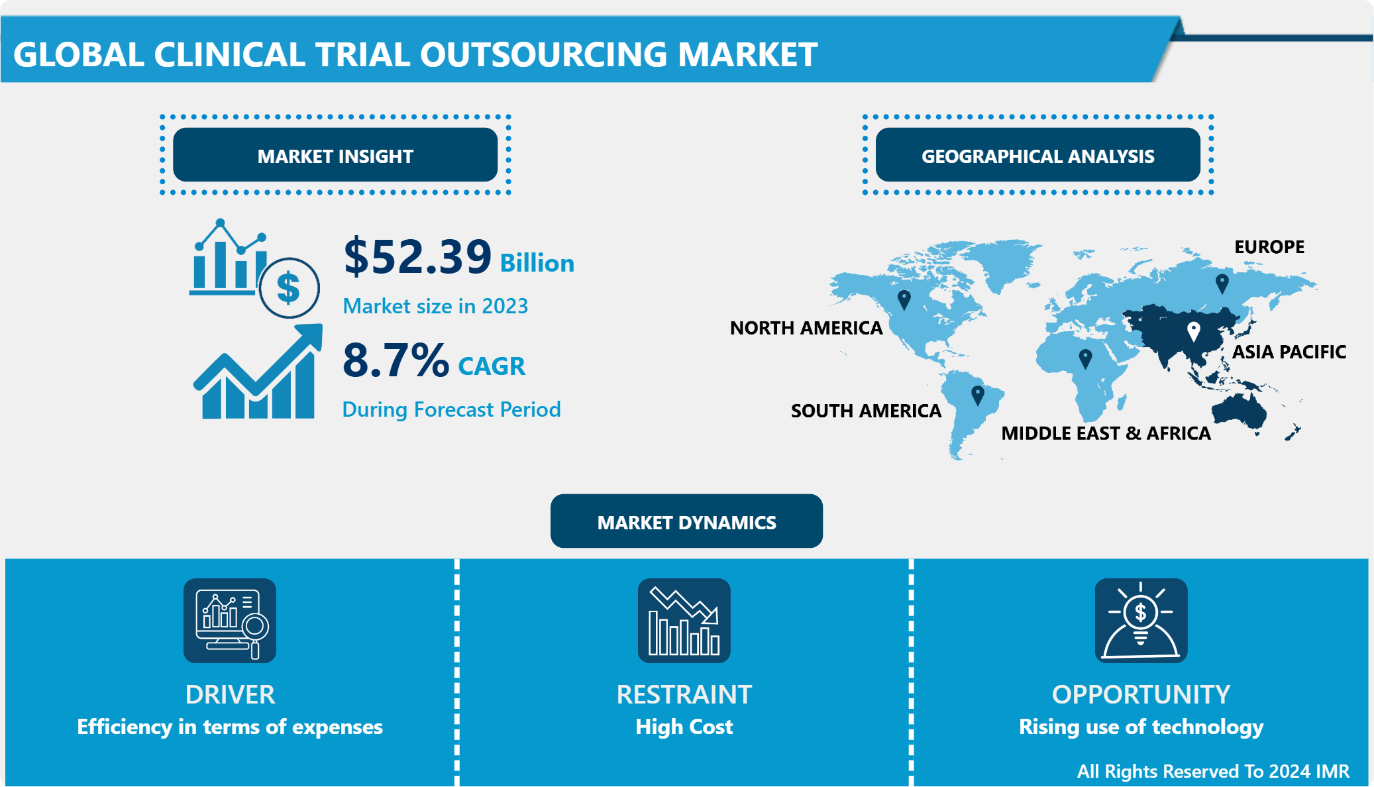
Introspective Market Research is thrilled to announce the release of its newest report, "Clinical Trial Outsourcing Market." This comprehensive analysis reveals that the global Clinical Trial Outsourcing market, valued at USD 52.39 Billion in 2023, is on a trajectory of significant growth, projected to reach USD 111.01 Billion by 2032. This upward momentum corresponds to a robust CAGR of 8.7% over the forecast period from 2024 to 2032.
Clinical trial outsourcing entails collaborating with an external organization to oversee some or all of the clinical trial tasks. This method is commonly used in clinical studies, helping organizations to lower expenses, boost effectiveness, and concentrate on their core abilities. By assigning duties like data handling, recruiting patients, ensuring regulatory compliance, and monitoring, companies can optimize their operations by utilizing the skills of specialized service providers. Companies aiming to conduct efficient clinical trials can benefit from outsourcing, which allows for faster timelines, resource optimization, and compliance with industry standards without stretching internal capabilities.
Outsourcing clinical trials provides multiple advantages that improve the overall efficiency and effectiveness of research endeavors. An important benefit is the cost-effectiveness since organizations only pay for the services and expertise they require, cutting out unnecessary expenses. Utilizing cutting-edge technology enables virtual trials, simplifying procedures and enhancing accessibility. Having experienced service providers to guide through intricate regulations minimizes risks and is a critical benefit in ensuring regulatory compliance. Moreover, outsourcing can enhance the standard of research, guaranteeing greater precision and dependability in findings. Having access to experienced clinical service providers results in receiving consistent support, which improves the implementation and results of trials.
This method also aids in decreasing cycle times, speeding up the development process while upholding quality. Furthermore, companies save on administrative expenses by assigning various operational duties to the outsourcing partner. By outsourcing non-essential tasks, businesses can enhance their focus on key strategic activities, leading to increased innovation and growth. Outsourcing also results in higher efficiency because specialized providers improve processes and results. Crucially, it enables businesses to manage expenses, offering a consistent financial framework for costs associated with trials. These benefits make outsourcing a crucial tactic for contemporary clinical research, achieving top-notch outcomes in a timely manner and complementing company goals.
Download Sample 250 Pages of Clinical Trial Outsourcing Market Report@ https://introspectivemarketresearch.com/request/16737
Key Industry Insights
Efficiency in terms of expenses
Managing clinical trials internally can demand a lot of resources, including substantial investments in infrastructure, staff, and technology. Hiring CROs offers an affordable option, as they spread out their resources among various projects, reducing the total trial cost for customers. Companies also steer clear of the prolonged financial stress related to upholding underutilized trial facilities.
Internationalization of Clinical Trials
Due to the growing need to test medications on a variety of populations for regulatory approval and increased market representation, clinical trials are expanding globally. CROs that have established presence in Asia, Eastern Europe, and Latin America offer local knowledge and access to sizable patient populations, leading to quicker recruitment and cost savings.
Expertise accessibility
CROs are experts in managing clinical trials, with expertise in areas such as trial design, implementation, data handling, and compliance with regulations. Specialized knowledge and experience are especially beneficial in intricate fields such as oncology or rare diseases, as they can greatly impact the results of trials.
Complex regulations
Clinical trials need to comply with different regulations in various countries, posing challenges for companies not familiar with local requirements. Contract Research Organizations (CROs) are skilled in managing these intricacies, guaranteeing adherence to local and international regulations like FDA, EMA, or PMDA policies, reducing the chances of regulatory setbacks or refusals.
Emphasize on Key Strengths.
Pharmaceutical and biotech companies strive to optimize their research and development endeavors through the exploration and creation of novel treatments. Through the use of outsourcing for the operational parts of trials, companies are able to focus on innovation and strategic goals, as CROs take care of the logistical and regulatory challenges.
"Research made simple and affordable – Trusted Research Tailored just for you – IMR Knowledge Cluster"
https://www.imrknowledgecluster.com/
What cost-related challenges affect the clinical trial outsourcing market?
The clinical trial outsourcing market is confronted with major challenges because of the expensive nature of clinical trials, which serve as primary obstacles. Even though outsourcing is a cheaper option than conducting operations in-house, the increasing costs of clinical trials still pose a significant challenge. Patient recruitment difficulties, sophisticated diagnostic and monitoring tools, and regulatory obligations contribute to the rising expenses. Enrolling patients can be very expensive, since locating suitable participants quickly usually involves significant efforts to reach out and offer incentives. In the same way, incorporating cutting-edge technologies to enhance trial efficiency and data accuracy requires significant upfront investments and ongoing maintenance expenses.
Another limitation is the presence of undisclosed expenses due to mismatched expectations between sponsors and outsourcing collaborators. Unexpected financial burdens can arise from changes in trial scope, unforeseen complexities, or additional services. If a contract research organization (CRO) miscalculates trial complexity or if protocol amendments occur, sponsors might encounter unexpected expenses. These additional expenses not just inflate total trial costs but also put a strain on relationships between sponsors and service providers. Therefore, although outsourcing provides benefits such as worldwide knowledge and operational effectiveness, the increasing expenses and undisclosed financial dangers are still major obstacles to market expansion, necessitating thorough preparation and open communication to address.
Key Trends of the Clinical Trial Outsourcing Market
Rising use of technology
The field of clinical trials is quickly adopting technology such as AI, machine learning, and e-clinical tools. AI is employed in patient enrollment, analyzing data, and forecasting results to make trials more efficient. E-clinical platforms allow for monitoring and collecting data from a distance, which helps cut down on logistical expenses. Telemedicine improves both the availability and involvement of patients. Transitioning to digital solutions enhances the efficiency, accessibility, and speeds up timelines for trials. Utilizing technology can assist in handling extensive datasets, enhancing decision-making, and backing decentralized trials, resulting in more efficient, affordable, and patient-focused clinical trials.
Clinical Trials' decentralization
DCTs are changing trial designs by enabling patients to take part from a distance. These experiments utilize telemedicine, mobile health applications, and electronic patient-reported outcomes (ePRO) to gather information without the need for in-person appointments. DCTs enhance patient convenience, recruitment, and retention by minimizing travel and logistical obstacles. This model allows for greater patient diversity and inclusion by making it simple for individuals from various geographic areas to participate. This transition was sped up by the COVID-19 pandemic, demonstrating that remote trials are feasible. DCTs increase the flexibility, accessibility, and adaptability of clinical trials to meet modern healthcare demands.
Outsourcing in Developing Countries
Outsourcing clinical trials to developing countries such as India, China, and Latin America is becoming more prevalent because of reduced expenses, rapid enrollment, and varied patient demographics. These areas provide unexplored populations of patients for various illnesses, especially those that are not common in developed nations. Furthermore, developing countries have enhanced their regulatory structures to meet global standards. Clinical research organizations in these areas have the necessary resources to handle intricate studies. Pharmaceutical companies find it appealing to conduct trials in these markets due to lower costs, allowing them to reach a wider range of patients without exceeding their budgets.
Collaborative relationships with CROs for strategic purposes
Drug companies are establishing extended partnerships with Contract Research Organizations (CROs) in order to simplify the process of clinical trials. These partnerships offer a wide range of services, including study planning, regulatory compliance, data handling, and report generation. By collaborating with one CRO, businesses can take advantage of steady knowledge, simplifying processes and guaranteeing quicker results. These collaborations enable better cost control, superior trial quality, and faster decision-making. CROs offer expertise in managing various global trials, guaranteeing adherence to local laws, and enhancing overall trial outcomes, all while easing the workload for pharmaceutical firms.
How can sponsors ensure consistent quality in global clinical trials with multiple CROs?
Maintaining uniform quality across various Contract Research Organizations (CROs) or service providers during worldwide clinical trials is a difficult task. Differences in operational standards, training, and expertise among providers can lead to varying outcomes, especially when outsourcing to countries with less experience in clinical trials. This difference could affect the precision, dependability, and general authenticity of clinical information.
One of the main difficulties lies in the differences in regulatory frameworks, ethical guidelines, and healthcare infrastructure among countries. CROs in less established areas may lack strong systems or adequately trained staff to handle the intricacies of clinical trials, resulting in irregular adherence to protocols and standards. Furthermore, communication may be further complicated by language barriers, cultural distinctions, and disparities in local regulations during the execution of uniform trial procedures.
Effective monitoring is essential in order to deal with these problems. This involves conducting routine audits, implementing clear and consistent guidelines, and utilizing technology for tracking data in real-time. Implementing centralized supervision and training programs for local teams and CRO staff can guarantee that trials are carried out at consistent high quality levels, no matter where they take place. By implementing rigorous quality control measures and promoting cooperation among global partners, sponsors can reduce risks and uphold the credibility of clinical trial findings.
Do you need any industry insights on Clinical Trial Outsourcing Market Make an enquiry now >> https://introspectivemarketresearch.com/inquiry/16737
Key Manufacturers
Identify the main players and organizations in a particular industry or market that have a strong impact on its dynamics. Recognizing these important individuals is crucial for grasping competitive positioning, market trends, and strategic opportunities.
- Parexel (Us)
- Iqvia (Us)
- Syneos Health (Us)
- Icon Plc(Us)
- Medidata Solutions (Us)
- PRA Health Sciences (Us)
- Covance (Us)
- Charles River Laboratories (Us)
- Catalent (Us)
- Emmes Company (Us)
- Syneos Health (Us)
- Fortrea Inc. (Us)
- Advanced Clinical (Us)
- Thermo Fisher Scientific Inc. (Us)
- Frontage Labs (Us)
- Acm Global Laboratories (Us)
- Worldwide Clinical Trials (Us)
- CTI Clinical Trial & Consulting (Us)
- Firma Clinical Research (Us)
- Celerion (Us)
- Boehringer Ingelheim International Gmbh (Europe)
- Pharmaserv International (Germany)
- Aptiv Solutions (France)
- Dove Quality Solutions (Uk)
- Clinigen Group (Uk) and Other Key Players
In October 2023, NAMSA, a top global MedTech Contract Research Organization (CRO) that provides comprehensive market access services, and TERUMO, a worldwide frontrunner in medical technology, have finalized a strategic outsourcing partnership. The goal of this partnership was to speed up the global regulatory approval and commercialization of Terumo's product lineup. By collaborating with NAMSA, TERUMO's products were able to gain approval in different global markets more efficiently,due to the pivotal role played by NAMSA's clinical research, testing, and consulting services in supporting regulatory processes.
In February 2023, PCM Trials, based in Denver and the largest independent provider of mobile research nurse visits for decentralized clinical trials (DCTs), announced the acquisition of EmVenio Research, located in Durham, North Carolina. EmVenio, the largest provider of community-based clinical trial sites served with mobile research units, was integrated into PCM Trials' operations. This acquisition enhanced both companies' abilities to recruit and retain diverse populations for clinical research studies, a key requirement for regulatory approval.
Key Segments of Market Report
By Service Type:
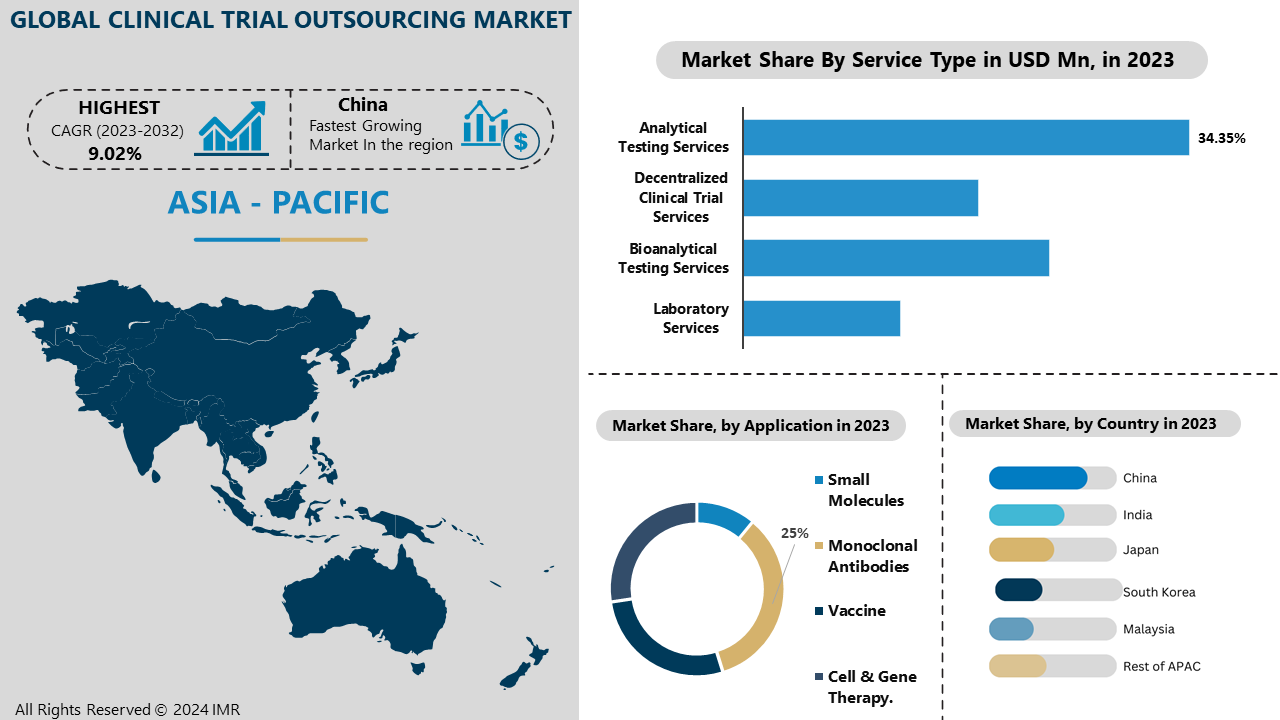
The Analytical Testing Services division is vital for verifying the safety, quality, and effectiveness of medications and substances in clinical trials. This section covers services such as developing methods, validating analytics, analyzing HPLC-MS, profiling impurities, conducting stability studies, and testing batch releases. These services are crucial in evaluating the quality, strength, and cleanliness of medication items, making sure they comply with regulations before being distributed for sale. Analytical testing services assist in reducing risks, enhancing the development process, and verifying product safety for consumer use due to the growing need for precise and dependable data in clinical trials.
By Application:
Monoclonal antibodies (mAbs) are playing a crucial role in combatting SARS-CoV-2, providing temporary protection and acting as a possible link to vaccines in the initial phases of the COVID-19 outbreak. Their capacity to focus on particular viral elements has positioned them as a crucial treatment option in clinical trials, including those supported by Regeneron Pharmaceuticals in partnership with the National Institute of Allergy and Infectious Diseases (NIAID). The increasing significance of mAbs in these trials is a key factor in their leading position in the Clinical Trial Outsourcing Market, with pharmaceutical companies and CROs prioritizing the advancement, evaluation, and marketing of monoclonal antibody therapies. This emerging pattern highlights the increasing need for specialized outsourcing services in clinical trials, especially in biologics and vaccine development sectors.
By Region:
The Clinical Trial Outsourcing Market in the Asia Pacific (APAC) region is being led by cost-effective labor, large patient populations, and highly skilled clinical research professionals, giving it strategic advantages. Countries such as India, China, and Japan provide a variety of well-regulated settings for clinical trials. This attracts pharmaceutical and biotechnology firms looking for cost-effective, high-quality trial services, as opposed to Western regions. Moreover, the APAC region's rising healthcare infrastructure, supportive government policies, and growing utilization of advanced technologies like artificial intelligence and data analytics in clinical trials are enhancing its strong position in the worldwide market. More multinational pharmaceutical companies are anticipated to keep seeking outsourced clinical trial services from APAC, leading to a continued trend.
If you require any specific information that is not covered currently, we will provide the same as a part of the customization >> https://introspectivemarketresearch.com/custom-research/16737
Comprehensive Offerings:
Market Size and Competitive Landscape (2017–2023): A detailed analysis of the market size and the competitive environment over recent years.
Pricing Trends and Regional Price Analysis (2017–2023): A review of past pricing trends and data across different regions.
Market Size, Share, and Forecast by Segment (2024–2032): Forecasts and insights into the market size, share, and expected growth by segment.
Market Dynamics: A comprehensive analysis of growth drivers, challenges, opportunities, and major trends, with a focus on regional variations.
Trend Analysis: Evaluation of emerging trends influencing the market environment.
Trade Overview: An examination of import and export patterns and their impact on market dynamics.
Market Segmentation: A detailed analysis of market segments and sub-segments, including a regional breakdown.
Competitive Landscape: Strategic profiles of key players in various regions, along with competitive benchmarking.
PESTLE Analysis: An evaluation of the market based on political, economic, social, technological, legal, and environmental factors.
Porter’s Five Forces Analysis: An examination of the competitive forces that affect the market.
Industry Value Chain Analysis: A look at the value chain to identify key stages and contributors.
Legal and Regulatory Framework by Region: An analysis of the legal environment and its implications for business operations.
Strategic Opportunities and SWOT Analysis: Identification of profitable business opportunities, along with a SWOT analysis.
Conclusion and Strategic Recommendations: Final insights and actionable recommendations for stakeholders.
Related Report Links:
Clinical Microbiology Market: Clinical Microbiology Market Size Was Valued at USD 4.5 Billion in 2023 and is Projected to Reach USD 7.3 Billion by 2032, Growing at a CAGR of 5.5% From 2024-2032.
Clinical Laboratory Tests Market: Clinical Laboratory Tests Market Size Was Valued at USD 114.89 Billion in 2023 and is Projected to Reach USD 265.41 Billion by 2032, Growing at a CAGR of 9.75% From 2024-2032.
Healthcare IT Market: Healthcare IT Market Size is Valued at USD 663.00 Billion in 2023, and is Projected to Reach USD 2482.50 Billion by 2032, Growing at a CAGR of 15.80% From 2024-2032.
Formulation Development Outsourcing Market: Formulation Development Outsourcing Market Size was valued at USD 26.8 Billion in 2023, and is Projected to Reach USD 58.6 Billion by 2032, Growing at a CAGR of 9.1% From 2024-2032.
Clinical Laboratory Services Market: Clinical Laboratory Services Market Size is Valued at USD 233.24 Billion in 2023 and is Projected to Reach USD 317.88 Billion by 2032, Growing at a CAGR of 3.50% From 2024-2032.
Clinical Perinatal Software Market: Clinical Perinatal Software Market Size Was Valued at USD 537.02 Million in 2023 and is Projected to Reach USD 1089.72 Million by 2032, Growing at a CAGR of 8.18% From 2024-2032.
Medical Billing Outsourcing Market: Medical Billing Outsourcing Market Size Was Valued at USD 14.6 Billion in 2023, and is Projected to Reach USD 41.3 Billion by 2032, Growing at a CAGR of 12.2% From 2024-2032.
Clinical Data Management System (CDMS) Market: Global Clinical Data Management System (CDMS) Market size is expected to grow from USD 2.3 Billion in 2022 to USD 5.42 Billion by 2030, at a CAGR of 11.3% during the forecast period (2023–2030).
Smart Contracts in Healthcare Market: Smart Contracts in Healthcare Market Size is Valued at USD 2.31 Billion in 2023, and is Projected to Reach USD 6.18 Billion by 2032, Growing at a CAGR of 17.19% From 2024-2032.
Clinical Trial Management Software Market: Global Clinical Trial Management Software Market size is expected to grow from USD 1.83 Billion in 2023 to USD 6.09 Billion by 2032, at a CAGR of 14.3% during the forecast period (2024-2032).
About Us:
Introspective Market Research is a leading global market research firm that harnesses the power of big data and advanced analytics to provide strategic insights and consulting solutions. Our expertise enables clients to predict future market trends with accuracy. The IMR team is dedicated to helping businesses understand both historical and current market dynamics, offering a clear view of potential future developments. Through strong industry connections, we gain access to critical market data, allowing us to create reliable research tables and precise forecasts. Led by CEO Mrs. Swati Kalagate, who fosters a culture of excellence, we are committed to delivering high-quality data that drives our clients' business success.
Our reports are built upon comprehensive primary research, including interviews with key executives from leading companies across relevant industries. Our secondary research process includes thorough online and offline investigations, in-depth discussions with industry experts, and collaboration with seasoned analysts. This rigorous approach ensures the accuracy and reliability of the insights we provide, empowering our clients with the information they need to make informed decisions, stay ahead of market trends, and achieve sustainable growth in their respective sectors. At IMR, we are focused on delivering data that helps businesses navigate the complexities of evolving markets with confidence.
Learn more about the principles guiding our success through our exclusive MORE Principles videos, showcasing our commitment to excellence in market research and strategic insights. Check out the links below to dive into our exclusive content!
https://youtu.be/V7Un49NVT-o?si=uIzneNa4P8FU9ssx
https://youtu.be/QzGHKVl4tNo?si=ztitVhc4nxPukmjY
https://youtu.be/SE8DnyFT3c4?si=nVHNd7oBgnsHaYcm
https://youtu.be/26OGoPDYVBU?si=zinoPO1tFWsPK0Kg
https://youtu.be/rUg_-uXfSEo?si=iIlUynPBvy3iKAd8
Contact Us:
Canada Office
Introspective Market Research Private Limited, 138 Downes Street Unit 6203- M5E 0E4, Toronto, Canada.
APAC Office
Introspective Market Research Private Limited, Office No. 401, Saudamini Commercial Complex, Kothrud, Pune, India 411038
Ph no: +91-81800-96367 / +91-7410103736
Email: sales@introspectivemarketresearch.com
LinkedIn| Twitter| Facebook | Instagram
Ours Websites : https://introspectivemarketresearch.com | https://imrknowledgecluster.com/knowledge-cluster | https://imrtechsolutions.com | https://imrnewswire.com/ | https://marketnresearch.de |
