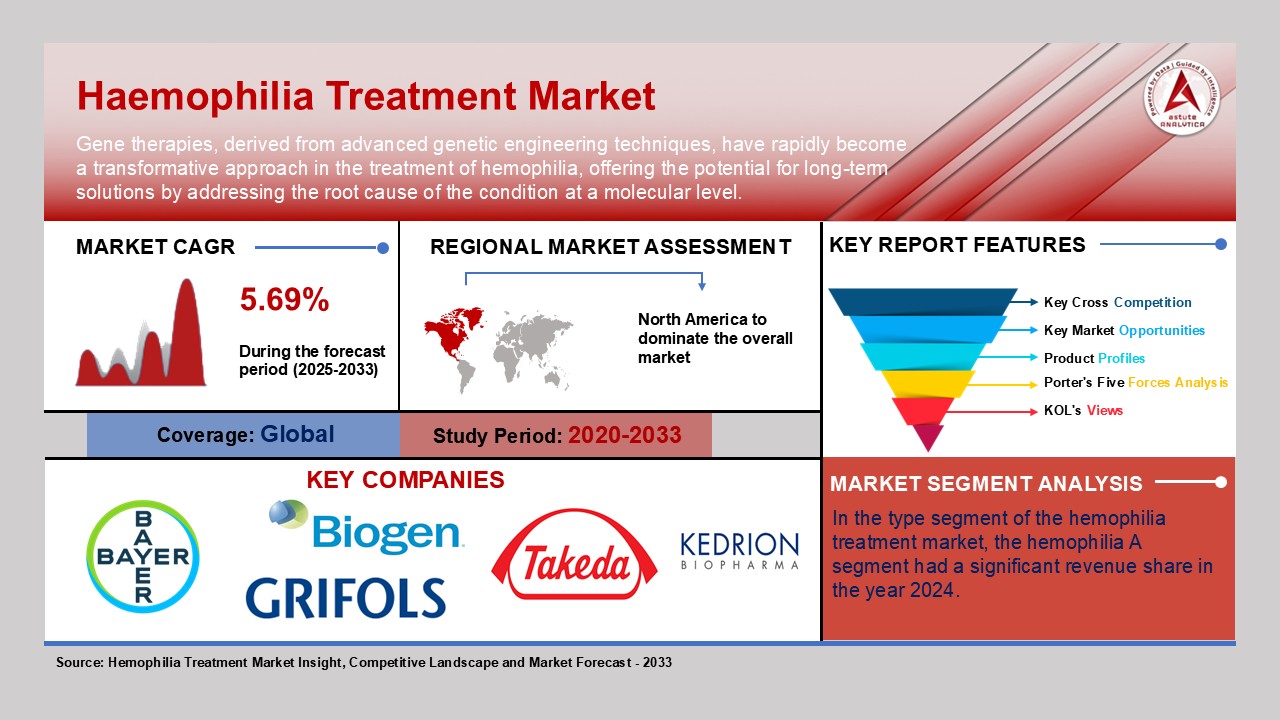
New Delhi, Jan. 31, 2025 (GLOBE NEWSWIRE) -- The global hemophilia treatment market is set for significant growth, with revenues expected to rise from USD 13.23 billion in 2024 to USD 21.48 billion by 2033, reflecting a strong CAGR of 5.7%. The global hemophilia treatment market is undergoing a transformative shift, driven by innovative therapies and growing clinical demand. Prophylactic treatments are becoming increasingly prominent, as patients and healthcare providers focus on reducing the frequency of bleeding episodes and improving overall quality of life. Notable advancements in gene therapies and recombinant clotting factors are revolutionizing patient care, offering more efficient and lasting solutions for hemophilia management. In the past 2 years, several new therapies have been approved, demonstrating rapid innovation in the market. Moreover, investments in research and development are accelerating the discovery of cutting-edge treatments, further propelling market growth. Research shows that prophylaxis with factor replacement drugs, plays a significant role in reducing the number of bleeds per year.
Request a sample and discover the recent advances in hemophilia treatment market @ https://www.astuteanalytica.com/request-sample/hemophilia-treatment-market
Notable approvals, such as the FDA’s green light for new therapies like Sevenfact, highlight the momentum behind next-generation treatments, which are shaping the future of hemophilia care. Analysts predict that the market will see a rise in novel drug introductions in the next few years, signaling strong future growth.
Despite the promising advancements, high treatment costs remain a significant challenge, particularly in emerging markets. In developed countries, treatment costs can reach up to $500,000 annually per patient per year, making access to these life-saving therapies difficult for many. This pricing barrier, coupled with inconsistent insurance coverage, continues to hinder broader adoption, especially in private healthcare systems. However, demand for hemophilia treatments remains strong, supported by increasing patient awareness, expanding healthcare infrastructure, and the growing prevalence of severe hemophilia cases worldwide.
As the market continues to evolve, stakeholders including manufacturers, healthcare providers, and research organizations are intensifying efforts to develop cost-effective, innovative solutions to meet the growing demand. The future of the hemophilia treatment market looks promising, with sustained R&D efforts and rising global adoption paving the way for continued growth.
Key Findings in Hemophilia Treatment Market
| Market Forecast (2033) | US$ 21.48 Billion |
| CAGR | 5.69% |
| Top Drivers | Rising Shift Towards Prophylactic Treatments |
| Growing Investments in R&D for Innovative Therapies | |
| Advancements in Gene Editing Technologies | |
| Top Trends | Growing adoption of Digital Health Technologies |
| Growing Role of Online Pharmacies in Treatment Access | |
| Strategic Partnerships and Collaborations in Drug Development | |
| Top Challenges | High Costs of Treatments of Hemophilia |
| Regulatory and Reimbursement Challenges | |
| Limited Access to Advanced Treatments in Emerging Markets |
Technological Innovation & Advancements: Revolutionary Advancements in Gene Editing and Digital Health Technologies Reshaping Hemophilia Treatment
The hemophilia treatment landscape is experiencing groundbreaking changes, driven by significant advancements in both gene editing technologies and digital health innovations, offering new opportunities for patients and healthcare providers. Gene therapy has emerged as the leading innovation for treating both hemophilia A and B, offering the potential for long-term, self-sustained clotting factor production through a single vector injection. As of June 2024, the European Association for Haemophilia and Allied Disorders (EAHAD) reports that 11 clinical trials are ongoing, involving more than 300 patients (202 for hemophilia A and 135 for hemophilia B). These therapies aim to elevate factor VIII or IX levels above 1% of the normal, thereby significantly reducing bleeding risks and eliminating the need for frequent infusions.
Recent milestones highlight the rapid progress in this area, with fidanacogene elaparvovec becoming the second gene therapy approved for hemophilia B in 2024, following etranacogene dezaparvovec-drlb's approval in 2022. These therapies are eliminating the need for lifelong prophylactic treatments, dramatically enhancing patient quality of life.
In parallel, the integration of digital health technologies is significantly enhancing self-management and care efficiency for hemophilia patients. Tools like mobile apps, telemedicine, and remote monitoring devices are improving patient outcomes by enabling individuals to log infusions, track bleeding episodes, and maintain direct communication with healthcare providers. Examples include HemMobile and Pfizer’s Hemophilia Health Tracker, which facilitate personalized care and better treatment adherence. A study found that 87.5% of patients prioritized infusion log documentation, 85.7% valued bleeding event tracking, and 71.4% emphasized secure data sharing with healthcare providers, reinforcing the demand for digital tools in hemophilia management. The rapid adoption of telehealth among patients has expanded access to remote consultations, facilitating timely treatment adjustments and continuous care.
The combined impact of gene therapy and digital health innovations is driving rapid market growth, offering a more efficient, accessible, and patient-centric approach to hemophilia care. As these technologies continue to evolve, the global demand for advanced hemophilia treatments is expected to soar, shaping a brighter future for hemophilia patients worldwide.
Inquire about this report before purchasing: https://www.astuteanalytica.com/inquire-before-purchase/hemophilia-treatment-market
Regional Growth Dynamics: Key Influences Shaping the haemophilia Treatment Market
The global hemophilia treatment market is experiencing a transformative shift, with regional dynamics playing a crucial role in shaping growth trajectories and treatment accessibility. As advancements in biotechnology, gene therapy, and digital health solutions accelerate, the demand for hemophilia treatments continues to surge across key regions, driven by varying healthcare infrastructures, government policies, and patient demographics. North America, Europe, and the Asia-Pacific region stand out as the primary markets, each contributing uniquely to the expansion and innovation of hemophilia care.
North America remains the dominant force in the global hemophilia treatment market, propelled by an advanced healthcare system, strong R&D investments, and the presence of leading pharmaceutical players. The region benefits from widespread insurance coverage, enabling broad patient access to cutting-edge therapies such as gene therapies. The United States leads in hemophilia research and treatment innovation, with companies like Pfizer, BioMarin, and Takeda at the forefront of developing next-generation solutions. The high adoption of digital health technologies, including AI-driven monitoring devices and telemedicine platforms, further strengthens North America’s position as a leader in hemophilia management.
Europe follows closely behind, driven by well-established healthcare systems, government-backed initiatives, and a strong focus on research and development. For instance, The Kreuth Initiative in Europe promotes optimal hemophilia care through universal access to prophylaxis, the use of extended half-life coagulation factor concentrates, and maintaining plasma factor levels of 3-5%. Leading countries like Germany, the UK, and France benefit from early therapy adoption and strategic biotech investments. The European Hemophilia Consortium (EHC) has been key in improving patient care access, advocating for comprehensive guidelines, and fostering collaboration between stakeholders.
Meanwhile, the Asia-Pacific region is poised for the fastest growth in the hemophilia treatment market, fueled by an increasing patient population, rising awareness, and improving healthcare accessibility. Countries such as Japan and China are leading this expansion, with substantial government investments in healthcare infrastructure and a growing emphasis on early diagnosis and treatment. For instance, the Japanese Hemophilia Network Committee (JHNC), organized by the Japanese Society of Thrombosis and Hemostasis, consists of hemophilia care centers and institutes. It aims to deliver comprehensive, personalized care to patients in collaboration with these centers. China, with its rapidly evolving pharmaceutical sector, is making strides in producing cost-effective recombinant therapies and biosimilars, improving affordability and accessibility. The region’s growing emphasis on public-private partnerships, coupled with the integration of telemedicine and AI-driven diagnostic tools, positions Asia-Pacific as a crucial driver of future market expansion.
Latin America and the Middle East & Africa are making gradual progress in hemophilia treatment. While Brazil and Argentina improve access to therapies, challenges like funding and logistics hinder progress. Similarly, in the Middle East & Africa, healthcare improvements and government initiatives are expanding access, but economic constraints and lack of awareness remain barriers to broader adoption of advanced treatments.
Key Players and Recent Developments in Hemophilia Treatment
Several prominent companies, including Pfizer Inc., CSL Behring, Takeda Pharmaceuticals, and Novo Nordisk, Sanofi, and Roche are driving innovation in the hemophilia treatment market. These companies are pioneering new therapies, such as gene therapies and subcutaneous treatments, for hemophilia A and B. For instance, Pfizer’s HYMPAVZI is the first anti-TFPI treatment approved for hemophilia A and B in October 2024, offering a convenient weekly subcutaneous dosing.
Similarly, in June 2023, CSL Behring’s HEMGENIX, a gene therapy for hemophilia B, has shown impressive results, with 94% of patients experiencing a significant reduction in prophylactic treatment needs. Additionally, in April 2024, Pfizer’s BEQVEZ received FDA approval as a treatment for adults with moderate to severe hemophilia B.
These advancements are reshaping the hemophilia treatment landscape and improving patient outcomes. The continued development of therapies from these key players is expected to drive market growth and establish new standards of care in hemophilia management.
Future Outlook: Shaping the Future of Hemophilia Treatment Market
The hemophilia treatment market is poised for significant growth, fueled by groundbreaking innovations in gene therapies, personalized care, and convenient treatment options. The increasing prevalence of hemophilia, combined with advancements in targeted therapies, presents substantial opportunities for pharmaceutical companies, healthcare providers, and investors to redefine the treatment landscape. Industry experts foresee a rise in the development and approval of novel therapies, with a growing focus on gene therapies that offer the potential for long-term or permanent solutions to hemophilia A and B. Companies like Pfizer, CSL Behring, and Takeda are leading the charge in this space, with ongoing clinical trials and new product approvals, such as gene therapies and subcutaneous treatments, set to transform patient care. Additionally, the adoption of digital health technologies, including mobile apps, telemedicine, and remote monitoring devices, is expected to enhance treatment adherence, improve outcomes, and lower the burden on both patients and healthcare systems.
By 2030, the market will likely see broader integration of gene therapies, digital health tools, and advanced treatments into routine care, shaping a new era of hemophilia management. The success of these advancements will depend on affordability, accessibility, and regulatory support to ensure that patients worldwide benefit from the full potential of these transformative therapies.
Key Players in Haemophilia Treatment Market
- Bayer AG
- Biogen Inc.
- BioMarin Pharmaceutical Inc
- Takeda Pharmaceuticals
- CSL Behring LLC
- F. Hoffmann-La Roche AG
- Ferring B.V.
- Genentech, Inc. (Roche Holding AG)
- Grifols, S.A
- Kedrion S.p.A
- Medexus Pharmaceuticals Inc.
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Sanofi SA
- Swedish Orphan Biovitrum AB
- Takeda Pharmaceuticals
- Other Prominent Players
Segments Covered in The Report
By Type
- Hemophilia A
- Hemophilia B
- Hemophilia C
- Others
By Product
- Recombinant coagulation factor concentrates
- Plasma-derived coagulation factor concentrates
- Desmopressin
- Antifibrinolytic agents
- Gene therapy products
- Others
By Patient
- Pediatric
- 0 to 4 yrs
- 5 to 13 yrs
- 14 to18 yrs
- Adult
- 19 to 44 yrs
- 45+ yrs
By Treatment Type
- On-Demand Treatment
- Prophylactic Treatment
- Immune Tolerance Induction (ITI) Therapy
By Route of Administration
- Intravenous
- Subcutaneous
By End user
- Hospitals
- Specialty Clinics
- Home Care Settings
- Hemophilia Treatment Centers (HTCs)
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Geography
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- South America
For further information on the hemophilia treatment market, reach out @ https://www.astuteanalytica.com/industry-report/hemophilia-treatment-market
About Astute Analytica
Astute Analytica is a global analytics and advisory company which has built a solid reputation in a short period, thanks to the tangible outcomes we have delivered to our clients. We pride ourselves in generating unparalleled, in depth and uncannily accurate estimates and projections for our very demanding clients spread across different verticals. We have a long list of satisfied and repeat clients from a wide spectrum including technology, healthcare, chemicals, semiconductors, FMCG, and many more. These happy customers come to us from all across the Globe. They are able to make well calibrated decisions and leverage highly lucrative opportunities while surmounting the fierce challenges all because we analyze for them the complex business environment, segment wise existing and emerging possibilities, technology formations, growth estimates, and even the strategic choices available. In short, a complete package. All this is possible because we have a highly qualified, competent, and experienced team of professionals comprising of business analysts, economists, consultants, and technology experts. In our list of priorities, you-our patron-come at the top. You can be sure of best cost-effective, value-added package from us, should you decide to engage with us.
Contact Us:
Astute Analytica
Phone: +1-888 429 6757 (US Toll Free); +91-0120- 4483891 (Rest of the World)
For Sales Enquiries: sales@astuteanalytica.com
Website: https://www.astuteanalytica.com/
LinkedIn | Twitter | YouTube